Preclinical Models of Prostate Cancer: Patient-Derived Xenografts, Organoids, and Other Explant Models
- PMID: 29311126
- PMCID: PMC6071547
- DOI: 10.1101/cshperspect.a030536
Preclinical Models of Prostate Cancer: Patient-Derived Xenografts, Organoids, and Other Explant Models
Abstract
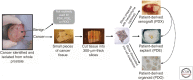
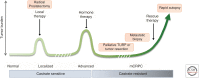
Prostate cancer remains a lethal disease. Preclinical cancer models that accurately represent the tumors of the patients they are intended to help are necessary to test potential therapeutic approaches and to better translate research discoveries. However, research in the prostate cancer field is hampered by the limited number of human cell lines and xenograft models, most of which do not recapitulate the human disease seen in the clinic today. This work reviews the recent advances in human patient-derived xenograft, organoid, and other explant models to address this need. In contrast to other tumor streams, the prostate cancer field is challenged by this approach, yet despite the limitations, patient-derived models remain an integral component of the preclinical testing pathway leading to better treatments for men with prostate cancer.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

Similar articles
-
A synopsis of prostate organoid methodologies, applications, and limitations.Prostate. 2020 May;80(6):518-526. doi: 10.1002/pros.23966. Epub 2020 Feb 21. Prostate. 2020. PMID: 32084293 Review.
-
Use of conditional reprogramming cell, patient derived xenograft and organoid for drug screening for individualized prostate cancer therapy: Current and future perspectives (Review).Int J Oncol. 2022 May;60(5):52. doi: 10.3892/ijo.2022.5342. Epub 2022 Mar 24. Int J Oncol. 2022. PMID: 35322860 Review.
-
Defining the challenges and opportunities for using patient-derived models in prostate cancer research.Prostate. 2024 May;84(7):623-635. doi: 10.1002/pros.24682. Epub 2024 Mar 7. Prostate. 2024. PMID: 38450798 Review.
-
High-Throughput Imaging Assay for Drug Screening of 3D Prostate Cancer Organoids.SLAS Discov. 2021 Oct;26(9):1107-1124. doi: 10.1177/24725552211020668. Epub 2021 Jun 11. SLAS Discov. 2021. PMID: 34111999 Free PMC article.
-
A PDX/Organoid Biobank of Advanced Prostate Cancers Captures Genomic and Phenotypic Heterogeneity for Disease Modeling and Therapeutic Screening.Clin Cancer Res. 2018 Sep 1;24(17):4332-4345. doi: 10.1158/1078-0432.CCR-18-0409. Epub 2018 May 10. Clin Cancer Res. 2018. PMID: 29748182 Free PMC article.
Cited by
-
Targeted therapy of the AKT kinase inhibits esophageal squamous cell carcinoma growth in vitro and in vivo.Int J Cancer. 2019 Aug 15;145(4):1007-1019. doi: 10.1002/ijc.32285. Epub 2019 Apr 3. Int J Cancer. 2019. PMID: 30887517 Free PMC article.
-
1,25-Dihydroxyvitamin D3 Suppresses Prognostic Survival Biomarkers Associated with Cell Cycle and Actin Organization in a Non-Malignant African American Prostate Cell Line.Biology (Basel). 2024 May 15;13(5):346. doi: 10.3390/biology13050346. Biology (Basel). 2024. PMID: 38785827 Free PMC article.
-
PDX: Moving Beyond Drug Screening to Versatile Models for Research Discovery.J Endocr Soc. 2020 Sep 12;4(11):bvaa132. doi: 10.1210/jendso/bvaa132. eCollection 2020 Nov 1. J Endocr Soc. 2020. PMID: 33094211 Free PMC article. Review.
-
The Role of ERα and ERβ in Castration-Resistant Prostate Cancer and Current Therapeutic Approaches.Biomedicines. 2023 Mar 9;11(3):826. doi: 10.3390/biomedicines11030826. Biomedicines. 2023. PMID: 36979805 Free PMC article. Review.
-
Addressing Patient Specificity in the Engineering of Tumor Models.Front Bioeng Biotechnol. 2019 Sep 12;7:217. doi: 10.3389/fbioe.2019.00217. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31572718 Free PMC article. Review.
References
-
- Alsop K, Thorne H, Sandhu S, Hamilton A, Mintoff C, Christie E, Spruyt O, Williams S, McNally O, Mileshkin L, et al. 2016. An effective community based model of rapid autopsy in end-stage cancer patients. Nat Biotechnol 34: 1010–1014. - PubMed
-
- Berner A, Henkel J, Woodruff MA, Saifzadeh S, Kirby G, Zaiss S, Gohlke J, Reichert JC, Nerlich M, Schuetz MA, et al. 2015. Scaffold-cell bone engineering in a validated preclinical animal model: Precursors vs differentiated cell source. J Tissue Eng Regen Med 11: 2081–2089. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
