A tetravalent virus-like particle vaccine designed to display domain III of dengue envelope proteins induces multi-serotype neutralizing antibodies in mice and macaques which confer protection against antibody dependent enhancement in AG129 mice
- PMID: 29309412
- PMCID: PMC5774828
- DOI: 10.1371/journal.pntd.0006191
A tetravalent virus-like particle vaccine designed to display domain III of dengue envelope proteins induces multi-serotype neutralizing antibodies in mice and macaques which confer protection against antibody dependent enhancement in AG129 mice
Abstract
Background: Dengue is one of the fastest spreading vector-borne diseases, caused by four antigenically distinct dengue viruses (DENVs). Antibodies against DENVs are responsible for both protection as well as pathogenesis. A vaccine that is safe for and efficacious in all people irrespective of their age and domicile is still an unmet need. It is becoming increasingly apparent that vaccine design must eliminate epitopes implicated in the induction of infection-enhancing antibodies.
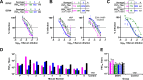
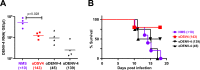
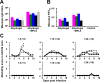
Methodology/principal findings: We report a Pichia pastoris-expressed dengue immunogen, DSV4, based on DENV envelope protein domain III (EDIII), which contains well-characterized serotype-specific and cross-reactive epitopes. In natural infection, <10% of the total neutralizing antibody response is EDIII-directed. Yet, this is a functionally relevant domain which interacts with the host cell surface receptor. DSV4 was designed by in-frame fusion of EDIII of all four DENV serotypes and hepatitis B surface (S) antigen and co-expressed with unfused S antigen to form mosaic virus-like particles (VLPs). These VLPs displayed EDIIIs of all four DENV serotypes based on probing with a battery of serotype-specific anti-EDIII monoclonal antibodies. The DSV4 VLPs were highly immunogenic, inducing potent and durable neutralizing antibodies against all four DENV serotypes encompassing multiple genotypes, in mice and macaques. DSV4-induced murine antibodies suppressed viremia in AG129 mice and conferred protection against lethal DENV-4 virus challenge. Further, neither murine nor macaque anti-DSV4 antibodies promoted mortality or inflammatory cytokine production when passively transferred and tested in an in vivo dengue disease enhancement model of AG129 mice.
Conclusions/significance: Directing the immune response to a non-immunodominant but functionally relevant serotype-specific dengue epitope of the four DENV serotypes, displayed on a VLP platform, can help minimize the risk of inducing disease-enhancing antibodies while eliciting effective tetravalent seroconversion. DSV4 has a significant potential to emerge as a safe, efficacious and inexpensive subunit dengue vaccine candidate.
Conflict of interest statement
All authors have declared that no competing interests exist. Further, LJW, MMM and REJ, who were employees of Global Vaccines, Inc. (a not-for-profit vaccine company), at the time of this work, declare that neither any of them nor the company has any proprietary or monetary interest in the DSV4 vaccine.
Figures



Similar articles
-
Dengue envelope-based 'four-in-one' virus-like particles produced using Pichia pastoris induce enhancement-lacking, domain III-directed tetravalent neutralising antibodies in mice.Sci Rep. 2018 Jun 5;8(1):8643. doi: 10.1038/s41598-018-26904-5. Sci Rep. 2018. PMID: 29872153 Free PMC article.
-
Virus-like particles derived from Pichia pastoris-expressed dengue virus type 1 glycoprotein elicit homotypic virus-neutralizing envelope domain III-directed antibodies.BMC Biotechnol. 2016 Jun 14;16(1):50. doi: 10.1186/s12896-016-0280-y. BMC Biotechnol. 2016. PMID: 27301568 Free PMC article.
-
Virus-like particles displaying envelope domain III of dengue virus type 2 induce virus-specific antibody response in mice.Vaccine. 2013 Jan 30;31(6):873-8. doi: 10.1016/j.vaccine.2012.12.016. Epub 2012 Dec 20. Vaccine. 2013. PMID: 23261049
-
Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine.Front Cell Infect Microbiol. 2020 Oct 22;10:572681. doi: 10.3389/fcimb.2020.572681. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33194810 Free PMC article. Review.
-
A recombinant, chimeric tetravalent dengue vaccine candidate based on a dengue virus serotype 2 backbone.Expert Rev Vaccines. 2016;15(4):497-508. doi: 10.1586/14760584.2016.1128328. Epub 2016 Feb 22. Expert Rev Vaccines. 2016. PMID: 26635182 Review.
Cited by
-
Interaction Between Virus-Like Particles (VLPs) and Pattern Recognition Receptors (PRRs) From Dendritic Cells (DCs): Toward Better Engineering of VLPs.Front Immunol. 2020 Jun 9;11:1100. doi: 10.3389/fimmu.2020.01100. eCollection 2020. Front Immunol. 2020. PMID: 32582186 Free PMC article. Review.
-
Plant-made dengue virus-like particles produced by co-expression of structural and non-structural proteins induce a humoral immune response in mice.Plant Biotechnol J. 2021 Apr;19(4):745-756. doi: 10.1111/pbi.13501. Epub 2020 Nov 22. Plant Biotechnol J. 2021. PMID: 33099859 Free PMC article.
-
Evaluation of cell-surface displayed synthetic consensus dengue EDIII cells as a potent oral vaccine candidate.Microb Cell Fact. 2018 Sep 14;17(1):146. doi: 10.1186/s12934-018-0994-8. Microb Cell Fact. 2018. PMID: 30217208 Free PMC article.
-
Novel expression of coat proteins from thermophilic bacteriophage ΦIN93 and evaluation for assembly into virus-like particles.Protein Expr Purif. 2021 Nov;187:105932. doi: 10.1016/j.pep.2021.105932. Epub 2021 Jun 29. Protein Expr Purif. 2021. PMID: 34214599 Free PMC article.
-
Identifying Key Drivers of Efficient B Cell Responses: On the Role of T Help, Antigen-Organization, and Toll-like Receptor Stimulation for Generating a Neutralizing Anti-Dengue Virus Response.Vaccines (Basel). 2024 Jun 14;12(6):661. doi: 10.3390/vaccines12060661. Vaccines (Basel). 2024. PMID: 38932390 Free PMC article.
References
-
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature 2013; 496: 504–507. doi: 10.1038/nature12060 - DOI - PMC - PubMed
-
- World Health Organization. Sustaining the drive to overcome the global impact of neglected tropical diseases: Second WHO report on Neglected tropical diseases WHO, 2013; WHO/HTM/NTD/2013.1.
-
- Lindenbach BD, Murray CL, Thiel HJ, Rice CM. Flaviviridae In: Knipe DM, Howley PM, editors-in-chief. Fields Virology, 6e. Philadelphia: Wolters Kluwer and Lippincott Williams & Wilkins; 2013. Pp. 712–746.
-
- Holmes EC, Twiddy SS. The origin, emergence and evolutionary genetics of dengue virus. Infection Genetic & Evolution 2003; 3: 19–28. - PubMed
-
- Rico-Hesse R. Molecular evolution and distribution of dengue viruses type 1 and 2 in nature. Virology 1990; 174: 479–493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

