Cellular Immune Correlates Preventing Disease Against Respiratory Syncytial Virus by Vaccination with Virus-Like Nanoparticles Carrying Fusion Proteins
- PMID: 29302248
- PMCID: PMC5749259
- DOI: 10.1166/jbn.2017.2341
Cellular Immune Correlates Preventing Disease Against Respiratory Syncytial Virus by Vaccination with Virus-Like Nanoparticles Carrying Fusion Proteins
Abstract
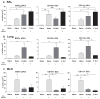
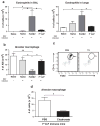
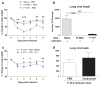
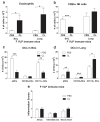
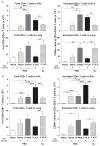
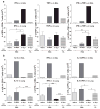
Cellular immune correlates conferring protection against respiratory syncytial virus (RSV) but preventing vaccine-enhanced respiratory disease largely remain unclear. We investigated cellular immune correlates that contribute to preventing disease against human respiratory syncytial virus (RSV) by nanoparticle vaccine delivery. Formalin-inactivated RSV (FI-RSV) vaccines and virus-like nanoparticles carrying RSV fusion proteins (F VLP) were investigated in mice. The FI-RSV vaccination caused severe weight loss and histopathology by inducing interleukin (IL)-4+, interferon (IFN)-γ+, IL-4+IFN-γ+ CD4+ T cells, eosinophils, and lung plasmacytoid dendritic cells (DCs), CD103+ DCs, and CD11b+ DCs. In contrast, the F VLP-immune mice induced protection against RSV without disease by inducing natural killer cells, activated IFN-γ+, and IFN-γ+ tumor necrosis factor (TNF)-α+ CD8+ T cells in the lung and bronchiolar airways during RSV infection but not disease-inducing DCs and effector T cells. Clodronate-mediated depletion studies provided evidence that alveolar macrophages that were present at high levels in the F VLP-immune mice play a role in modulating protective cellular immune phenotypes. There was an intrinsic difference between the F VLP and FI-RSV treatments in stimulating proinflammatory cytokines. The F VLP nanoparticle vaccination induced distinct innate and adaptive cellular subsets that potentially prevented lung disease after RSV infection.
Keywords: Alveolar Macrophages; Clodronate Liposome; Formalin-Inactivated RSV; Fusion Protein Nanoparticles; Respiratory Syncytial Virus (RSV).
Conflict of interest statement
Conflicts of Interest The authors declare no conflicts of interest.
Figures
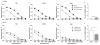

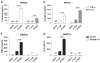
Similar articles
-
Virus-Like Particle Vaccine Containing the F Protein of Respiratory Syncytial Virus Confers Protection without Pulmonary Disease by Modulating Specific Subsets of Dendritic Cells and Effector T Cells.J Virol. 2015 Nov;89(22):11692-705. doi: 10.1128/JVI.02018-15. Epub 2015 Sep 9. J Virol. 2015. PMID: 26355098 Free PMC article.
-
Respiratory syncytial virus-like nanoparticle vaccination induces long-term protection without pulmonary disease by modulating cytokines and T-cells partially through alveolar macrophages.Int J Nanomedicine. 2015 Jul 14;10:4491-505. doi: 10.2147/IJN.S83493. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26203246 Free PMC article.
-
Novel Respiratory Syncytial Virus-Like Particle Vaccine Composed of the Postfusion and Prefusion Conformations of the F Glycoprotein.Clin Vaccine Immunol. 2016 Jun 6;23(6):451-9. doi: 10.1128/CVI.00720-15. Print 2016 Jun. Clin Vaccine Immunol. 2016. PMID: 27030590 Free PMC article.
-
Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy--a review.Virus Genes. 2006 Oct;33(2):235-52. doi: 10.1007/s11262-006-0064-x. Virus Genes. 2006. PMID: 16972040 Review.
-
Contribution of respiratory syncytial virus G antigenicity to vaccine-enhanced illness and the implications for severe disease during primary respiratory syncytial virus infection.Pediatr Infect Dis J. 2004 Jan;23(1 Suppl):S46-57. doi: 10.1097/01.inf.0000108192.94692.d2. Pediatr Infect Dis J. 2004. PMID: 14730270 Review.
Cited by
-
Immunopathology of RSV: An Updated Review.Viruses. 2021 Dec 10;13(12):2478. doi: 10.3390/v13122478. Viruses. 2021. PMID: 34960746 Free PMC article. Review.
-
Application of nanotechnology in drug delivery systems for respiratory diseases (Review).Mol Med Rep. 2021 May;23(5):325. doi: 10.3892/mmr.2021.11964. Epub 2021 Mar 24. Mol Med Rep. 2021. PMID: 33760125 Free PMC article. Review.
-
Learning from the past: development of safe and effective COVID-19 vaccines.Nat Rev Microbiol. 2021 Mar;19(3):211-219. doi: 10.1038/s41579-020-00462-y. Epub 2020 Oct 16. Nat Rev Microbiol. 2021. PMID: 33067570 Free PMC article.
-
Potential of Nanotechnology-based Formulations in Combating Pulmonary Infectious Diseases: A Current Scenario.Curr Pharm Des. 2022;28(42):3413-3427. doi: 10.2174/1381612829666221116143138. Curr Pharm Des. 2022. PMID: 36397631 Review.
-
Microparticle RSV Vaccines Presenting the G Protein CX3C Chemokine Motif in the Context of TLR Signaling Induce Protective Th1 Immune Responses and Prevent Pulmonary Eosinophilia Post-Challenge.Vaccines (Basel). 2022 Dec 5;10(12):2078. doi: 10.3390/vaccines10122078. Vaccines (Basel). 2022. PMID: 36560488 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
