HspB1 and Hsc70 chaperones engage distinct tau species and have different inhibitory effects on amyloid formation
- PMID: 29298892
- PMCID: PMC5827454
- DOI: 10.1074/jbc.M117.803411
HspB1 and Hsc70 chaperones engage distinct tau species and have different inhibitory effects on amyloid formation
Abstract
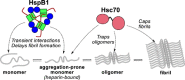
The microtubule-associated protein tau forms insoluble, amyloid-type aggregates in various dementias, most notably Alzheimer's disease. Cellular chaperone proteins play important roles in maintaining protein solubility and preventing aggregation in the crowded cellular environment. Although tau is known to interact with numerous chaperones, it remains unclear how these chaperones function mechanistically to prevent tau aggregation and how chaperones from different classes compare in terms of mechanism. Here, we focused on the small heat shock protein HspB1 (also known as Hsp27) and the constitutive chaperone Hsc70 (also known as HspA8) and report how each chaperone interacts with tau to prevent its fibril formation. Using fluorescence and NMR spectroscopy, we show that the two chaperones inhibit tau fibril formation by distinct mechanisms. HspB1 delayed tau fibril formation by weakly interacting with early species in the aggregation process, whereas Hsc70 was highly efficient at preventing tau fibril elongation, possibly by capping the ends of tau fibrils. Both chaperones recognized aggregation-prone motifs within the microtubule-binding repeat region of tau. However, HspB1 binding remained transient in both aggregation-promoting and non-aggregating conditions, whereas Hsc70 binding was significantly tighter under aggregation-promoting conditions. These differences highlight the fact that chaperones from different families play distinct but complementary roles in the prevention of pathological protein aggregation.
Keywords: 70 kilodalton heat shock protein (Hsp70); aggregation; amyloid; chaperone; small heat shock protein (sHsp); tau.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
Tau protein aggregates inhibit the protein-folding and vesicular trafficking arms of the cellular proteostasis network.J Biol Chem. 2019 May 10;294(19):7917-7930. doi: 10.1074/jbc.RA119.007527. Epub 2019 Apr 1. J Biol Chem. 2019. PMID: 30936201 Free PMC article.
-
Hsp40s play complementary roles in the prevention of tau amyloid formation.Elife. 2021 Aug 9;10:e69601. doi: 10.7554/eLife.69601. Elife. 2021. PMID: 34369377 Free PMC article.
-
Release of a disordered domain enhances HspB1 chaperone activity toward tau.Proc Natl Acad Sci U S A. 2020 Feb 11;117(6):2923-2929. doi: 10.1073/pnas.1915099117. Epub 2020 Jan 23. Proc Natl Acad Sci U S A. 2020. PMID: 31974309 Free PMC article.
-
Amyloidogenesis of Tau protein.Protein Sci. 2017 Nov;26(11):2126-2150. doi: 10.1002/pro.3275. Epub 2017 Sep 13. Protein Sci. 2017. PMID: 28833749 Free PMC article. Review.
-
Cellular factors modulating the mechanism of tau protein aggregation.Cell Mol Life Sci. 2015 May;72(10):1863-79. doi: 10.1007/s00018-015-1839-9. Epub 2015 Feb 11. Cell Mol Life Sci. 2015. PMID: 25666877 Free PMC article. Review.
Cited by
-
Exercise suppresses mouse systemic AApoAII amyloidosis through enhancement of the p38 MAPK signaling pathway.Dis Model Mech. 2022 Mar 1;15(3):dmm049327. doi: 10.1242/dmm.049327. Epub 2022 Mar 21. Dis Model Mech. 2022. PMID: 35099007 Free PMC article.
-
Tau protein aggregates inhibit the protein-folding and vesicular trafficking arms of the cellular proteostasis network.J Biol Chem. 2019 May 10;294(19):7917-7930. doi: 10.1074/jbc.RA119.007527. Epub 2019 Apr 1. J Biol Chem. 2019. PMID: 30936201 Free PMC article.
-
Mechanisms and pathology of protein misfolding and aggregation.Nat Rev Mol Cell Biol. 2023 Dec;24(12):912-933. doi: 10.1038/s41580-023-00647-2. Epub 2023 Sep 8. Nat Rev Mol Cell Biol. 2023. PMID: 37684425 Review.
-
Aberrant protein aggregation in amyotrophic lateral sclerosis.J Neurol. 2024 Aug;271(8):4826-4851. doi: 10.1007/s00415-024-12485-z. Epub 2024 Jun 13. J Neurol. 2024. PMID: 38869826 Review.
-
A weakened interface in the P182L variant of HSP27 associated with severe Charcot-Marie-Tooth neuropathy causes aberrant binding to interacting proteins.EMBO J. 2021 Apr 15;40(8):e103811. doi: 10.15252/embj.2019103811. Epub 2021 Mar 1. EMBO J. 2021. PMID: 33644875 Free PMC article.
References
-
- Dawson H. N., Ferreira A., Eyster M. V., Ghoshal N., Binder L. I., and Vitek M. P. (2001) Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J. Cell Sci. 114, 1179–1187 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

