Lipid droplet density alters the early innate immune response to viral infection
- PMID: 29293661
- PMCID: PMC5749834
- DOI: 10.1371/journal.pone.0190597
Lipid droplet density alters the early innate immune response to viral infection
Abstract
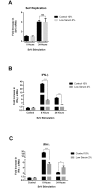
The cellular localisation of many innate signalling events following viral infection has yet to be elucidated, however there has been a few cases in which membranes of certain cellular organelles have acted as platforms to these events. Of these, lipid droplets (LDs) have recently been identified as signalling platforms for innate TLR7 and 9 signalling. Despite their wide range of similar roles in various metabolic pathways, LDs have been overlooked as potential platforms for antiviral innate signalling events. This study established an in vitro model to evaluate the efficiency of the early innate immune response in cells with reduced LD content to the viral mimics, dsDNA and dsRNA, and Sendai viral infection. Using RT-qPCR, the expression of IFN-β and IFN-λ was quantified following stimulation along with the expression of specific ISGs. Luciferase based assays evaluated the combined expression of ISRE-promoter driven ISGs under IFN-β stimulation. Cellular LD content did not alter the entry of fluorescently labelled viral mimics into cells, but significantly decreased the ability of both Huh-7 and HeLa cells to produce type I and III IFN, as well as downstream ISG expression, indicative of an impeded innate immune response. This observation was also seen during Sendai virus infection of HeLa cells, where both control and LD reduced cells replicated the virus to the same level, but a significantly impaired type I and III IFN response was observed in the LD reduced cells. In addition to altered IFN production, cells with reduced LD content exhibited decreased expression of specific antiviral ISGs: Viperin, IFIT-1 and OAS-1 under IFN-β stimulation; However the overall induction of the ISRE-promoter was not effected. This study implicates a role for LDs in an efficient early innate host response to viral infection and future work will endeavour to determine the precise role these important organelles play in induction of an antiviral response.
Conflict of interest statement
Figures
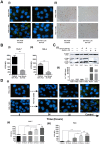
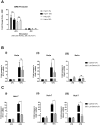
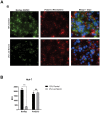
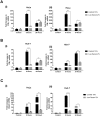
Similar articles
-
Intracellular lipid droplet accumulation occurs early following viral infection and is required for an efficient interferon response.Nat Commun. 2021 Jul 14;12(1):4303. doi: 10.1038/s41467-021-24632-5. Nat Commun. 2021. PMID: 34262037 Free PMC article.
-
Understanding Viral dsRNA-Mediated Innate Immune Responses at the Cellular Level Using a Rainbow Trout Model.Front Immunol. 2018 Apr 23;9:829. doi: 10.3389/fimmu.2018.00829. eCollection 2018. Front Immunol. 2018. PMID: 29740439 Free PMC article.
-
Lipid droplets participate in modulating innate immune genes in Ctenopharyngodon idella kidney cells.Fish Shellfish Immunol. 2019 May;88:595-605. doi: 10.1016/j.fsi.2019.03.032. Epub 2019 Mar 16. Fish Shellfish Immunol. 2019. PMID: 30890432
-
Innate and adaptive immune responses in HCV infections.J Hepatol. 2014 Nov;61(1 Suppl):S14-25. doi: 10.1016/j.jhep.2014.06.035. Epub 2014 Nov 3. J Hepatol. 2014. PMID: 25443342 Review.
-
Fish interferon-stimulated genes: The antiviral effectors.Dev Comp Immunol. 2016 Dec;65:218-225. doi: 10.1016/j.dci.2016.07.011. Epub 2016 Jul 19. Dev Comp Immunol. 2016. PMID: 27451256 Review.
Cited by
-
Viperin catalyzes methionine oxidation to promote protein expression and function of helicases.Sci Adv. 2019 Aug 28;5(8):eaax1031. doi: 10.1126/sciadv.aax1031. eCollection 2019 Aug. Sci Adv. 2019. PMID: 31489375 Free PMC article.
-
ISG15 Is a Novel Regulator of Lipid Metabolism during Vaccinia Virus Infection.Microbiol Spectr. 2022 Dec 21;10(6):e0389322. doi: 10.1128/spectrum.03893-22. Epub 2022 Dec 1. Microbiol Spectr. 2022. PMID: 36453897 Free PMC article.
-
Global Lipidome Profiling Revealed Multifaceted Role of Lipid Species in Hepatitis C Virus Replication, Assembly, and Host Antiviral Response.Viruses. 2023 Feb 7;15(2):464. doi: 10.3390/v15020464. Viruses. 2023. PMID: 36851679 Free PMC article.
-
Using Machine Learning to Identify Biomarkers Affecting Fat Deposition in Pigs by Integrating Multisource Transcriptome Information.J Agric Food Chem. 2022 Aug 24;70(33):10359-10370. doi: 10.1021/acs.jafc.2c03339. Epub 2022 Aug 11. J Agric Food Chem. 2022. PMID: 35953074 Free PMC article.
-
Aged lipid-laden microglia display impaired responses to stroke.EMBO Mol Med. 2023 Feb 8;15(2):e17175. doi: 10.15252/emmm.202217175. Epub 2022 Dec 21. EMBO Mol Med. 2023. PMID: 36541061 Free PMC article.
References
-
- Johnston JB, Nazarian SH, Natale R, McFadden G. Myxoma virus infection of primary human fibroblasts varies with cellular age and is regulated by host interferon responses. Virology. 2005;332(1):235–48. doi: 10.1016/j.virol.2004.11.030 - DOI - PubMed
-
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–95. doi: 10.1038/ni1112 - DOI - PubMed
-
- Kagan JC. Signaling organelles of the innate immune system. Cell. 2012;151(6):1168–78. doi: 10.1016/j.cell.2012.11.011 - DOI - PMC - PubMed
-
- West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11(6):389–402. doi: 10.1038/nri2975 - DOI - PMC - PubMed
-
- Kagan JC. Defining the subcellular sites of innate immune signal transduction. Trends Immunol. 2012;33(9):442–8. doi: 10.1016/j.it.2012.06.005 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

