Insights from human genetic studies of lung and organ fibrosis
- PMID: 29293091
- PMCID: PMC5749497
- DOI: 10.1172/JCI93556
Insights from human genetic studies of lung and organ fibrosis
Abstract
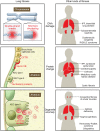
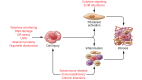
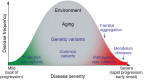
Genetic investigations of fibrotic diseases, including those of late onset, often yield unanticipated insights into disease pathogenesis. This Review focuses on pathways underlying lung fibrosis that are generalizable to other organs. Herein, we discuss genetic variants subdivided into those that shorten telomeres, activate the DNA damage response, change resident protein expression or function, or affect organelle activity. Genetic studies provide a window into the downstream cascade of maladaptive responses and pathways that lead to tissue fibrosis. In addition, these studies reveal interactions between genetic variants, environmental factors, and age that influence the phenotypic spectrum of disease. The discovery of forces counterbalancing inherited risk alleles identifies potential therapeutic targets, thus providing hope for future prevention or reversal of fibrosis.
Conflict of interest statement
Figures
Similar articles
-
Airway Mucosal Host Defense Is Key to Genomic Regulation of Cystic Fibrosis Lung Disease Severity.Am J Respir Crit Care Med. 2018 Jan 1;197(1):79-93. doi: 10.1164/rccm.201701-0134OC. Am J Respir Crit Care Med. 2018. PMID: 28853905 Free PMC article.
-
Gene expression profiles among murine strains segregate with distinct differences in the progression of radiation-induced lung disease.Dis Model Mech. 2017 Apr 1;10(4):425-437. doi: 10.1242/dmm.028217. Epub 2017 Jan 26. Dis Model Mech. 2017. PMID: 28130353 Free PMC article.
-
Telomeres in lung diseases.Prog Mol Biol Transl Sci. 2014;125:173-83. doi: 10.1016/B978-0-12-397898-1.00008-6. Prog Mol Biol Transl Sci. 2014. PMID: 24993703 Review.
-
The emerging roles of β-arrestins in fibrotic diseases.Acta Pharmacol Sin. 2015 Nov;36(11):1277-87. doi: 10.1038/aps.2015.74. Epub 2015 Sep 21. Acta Pharmacol Sin. 2015. PMID: 26388156 Free PMC article. Review.
-
Finding fibrosis genes: the lung.Methods Mol Med. 2005;117:293-313. doi: 10.1385/1-59259-940-0:293. Methods Mol Med. 2005. PMID: 16118459
Cited by
-
Familial Aggregation in Idiopathic Subglottic Stenosis.Otolaryngol Head Neck Surg. 2020 Nov;163(5):1011-1017. doi: 10.1177/0194599820935402. Epub 2020 Jun 30. Otolaryngol Head Neck Surg. 2020. PMID: 32600122 Free PMC article.
-
Regulator of telomere elongation helicase 1 gene and its association with malignancy.Cancer Rep (Hoboken). 2023 Jan;6(1):e1735. doi: 10.1002/cnr2.1735. Epub 2022 Oct 17. Cancer Rep (Hoboken). 2023. PMID: 36253342 Free PMC article. Review.
-
Insights into the Pathogenesis of Pulmonary Fibrosis from Genetic Diseases.Am J Respir Cell Mol Biol. 2022 Jul;67(1):20-35. doi: 10.1165/rcmb.2021-0557TR. Am J Respir Cell Mol Biol. 2022. PMID: 35294321 Free PMC article. Review.
-
TAZ is required for lung alveolar epithelial cell differentiation after injury.JCI Insight. 2019 Jun 18;5(14):e128674. doi: 10.1172/jci.insight.128674. JCI Insight. 2019. PMID: 31211697 Free PMC article.
-
Autoantibodies targeting telomere-associated proteins in systemic sclerosis.Ann Rheum Dis. 2021 Jul;80(7):912-919. doi: 10.1136/annrheumdis-2020-218918. Epub 2021 Jan 25. Ann Rheum Dis. 2021. PMID: 33495152 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

