Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD)
- PMID: 29286303
- PMCID: PMC5793257
- DOI: 10.3390/nu10010029
Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD)
Abstract
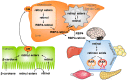
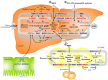
Vitamin A is required for important physiological processes, including embryogenesis, vision, cell proliferation and differentiation, immune regulation, and glucose and lipid metabolism. Many of vitamin A's functions are executed through retinoic acids that activate transcriptional networks controlled by retinoic acid receptors (RARs) and retinoid X receptors (RXRs).The liver plays a central role in vitamin A metabolism: (1) it produces bile supporting efficient intestinal absorption of fat-soluble nutrients like vitamin A; (2) it produces retinol binding protein 4 (RBP4) that distributes vitamin A, as retinol, to peripheral tissues; and (3) it harbors the largest body supply of vitamin A, mostly as retinyl esters, in hepatic stellate cells (HSCs). In times of inadequate dietary intake, the liver maintains stable circulating retinol levels of approximately 2 μmol/L, sufficient to provide the body with this vitamin for months. Liver diseases, in particular those leading to fibrosis and cirrhosis, are associated with impaired vitamin A homeostasis and may lead to vitamin A deficiency. Liver injury triggers HSCs to transdifferentiate to myofibroblasts that produce excessive amounts of extracellular matrix, leading to fibrosis. HSCs lose the retinyl ester stores in this process, ultimately leading to vitamin A deficiency. Non-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome and is a spectrum of conditions ranging from benign hepatic steatosis to non-alcoholic steatohepatitis (NASH); it may progress to cirrhosis and liver cancer. NASH is projected to be the main cause of liver failure in the near future. Retinoic acids are key regulators of glucose and lipid metabolism in the liver and adipose tissue, but it is unknown whether impaired vitamin A homeostasis contributes to or suppresses the development of NAFLD. A genetic variant of patatin-like phospholipase domain-containing 3 (PNPLA3-I148M) is the most prominent heritable factor associated with NAFLD. Interestingly, PNPLA3 harbors retinyl ester hydrolase activity and PNPLA3-I148M is associated with low serum retinol level, but enhanced retinyl esters in the liver of NAFLD patients. Low circulating retinol in NAFLD may therefore not reflect true "vitamin A deficiency", but rather disturbed vitamin A metabolism. Here, we summarize current knowledge about vitamin A metabolism in NAFLD and its putative role in the progression of liver disease, as well as the therapeutic potential of vitamin A metabolites.
Keywords: hepatic stellate cells; lipid metabolism; metabolic syndrome; non-alcoholic fatty liver disease; nuclear receptors; retinoic acid; retinol; retinol binding protein 4; retinyl esters; vitamin A.
Conflict of interest statement
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial or non-financial interest in the subject matter or materials described in this manuscript.
Figures
Similar articles
-
PNPLA3 has retinyl-palmitate lipase activity in human hepatic stellate cells.Hum Mol Genet. 2014 Aug 1;23(15):4077-85. doi: 10.1093/hmg/ddu121. Epub 2014 Mar 25. Hum Mol Genet. 2014. PMID: 24670599 Free PMC article.
-
Impaired Hepatic Vitamin A Metabolism in NAFLD Mice Leading to Vitamin A Accumulation in Hepatocytes.Cell Mol Gastroenterol Hepatol. 2021;11(1):309-325.e3. doi: 10.1016/j.jcmgh.2020.07.006. Epub 2020 Jul 19. Cell Mol Gastroenterol Hepatol. 2021. PMID: 32698042 Free PMC article.
-
PNPLA3 I148M Variant Influences Circulating Retinol in Adults with Nonalcoholic Fatty Liver Disease or Obesity.J Nutr. 2015 Aug;145(8):1687-91. doi: 10.3945/jn.115.210633. Epub 2015 Jul 1. J Nutr. 2015. PMID: 26136587 Free PMC article.
-
The interrelationship between bile acid and vitamin A homeostasis.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 May;1862(5):496-512. doi: 10.1016/j.bbalip.2017.01.007. Epub 2017 Jan 19. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 28111285 Review.
-
Molecular Mechanisms: Connections between Nonalcoholic Fatty Liver Disease, Steatohepatitis and Hepatocellular Carcinoma.Int J Mol Sci. 2020 Feb 23;21(4):1525. doi: 10.3390/ijms21041525. Int J Mol Sci. 2020. PMID: 32102237 Free PMC article. Review.
Cited by
-
Vitamin A, D, E, and K as Matrix Metalloproteinase-2/9 Regulators That Affect Expression and Enzymatic Activity.Int J Mol Sci. 2023 Dec 1;24(23):17038. doi: 10.3390/ijms242317038. Int J Mol Sci. 2023. PMID: 38069361 Free PMC article. Review.
-
Hepatitis A Leading to Severe Vitamin A Deficiency and Bitot's Spots in a Three-Year-Old Male Child: A Case Report.Cureus. 2024 Jan 7;16(1):e51821. doi: 10.7759/cureus.51821. eCollection 2024 Jan. Cureus. 2024. PMID: 38327965 Free PMC article.
-
Modulation of retinoid signaling: therapeutic opportunities in organ fibrosis and repair.Pharmacol Ther. 2020 Jan;205:107415. doi: 10.1016/j.pharmthera.2019.107415. Epub 2019 Oct 16. Pharmacol Ther. 2020. PMID: 31629008 Free PMC article. Review.
-
Effects of AM80 compared to AC261066 in a high fat diet mouse model of liver disease.PLoS One. 2019 Jan 24;14(1):e0211071. doi: 10.1371/journal.pone.0211071. eCollection 2019. PLoS One. 2019. PMID: 30677086 Free PMC article.
-
Understanding NAFLD: From Case Identification to Interventions, Outcomes, and Future Perspectives.Nutrients. 2023 Jan 29;15(3):687. doi: 10.3390/nu15030687. Nutrients. 2023. PMID: 36771394 Free PMC article. Review.
References
-
- Lo C.S., Wahlqvist M.L., Horie Y. Determination of retinoic acid and retinol at physiological concentration by HPLC in Caucasians and Japanese women. Asia Pac. J. Clin. Nutr. 1996;5:173–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

