MicroRNA-146 and cell trauma down-regulate expression of the psoriasis-associated atypical chemokine receptor ACKR2
- PMID: 29279330
- PMCID: PMC5827444
- DOI: 10.1074/jbc.M117.809780
MicroRNA-146 and cell trauma down-regulate expression of the psoriasis-associated atypical chemokine receptor ACKR2
Abstract
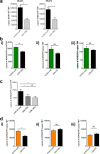
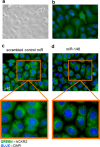
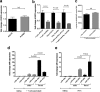
Chemokines are the principal regulators of leukocyte migration and are essential for initiation and maintenance of inflammation. Atypical chemokine receptor 2 (ACKR2) binds and scavenges proinflammatory CC-chemokines, regulates cutaneous T-cell positioning, and limits the spread of inflammation in vivo Altered ACKR2 function has been implicated in several inflammatory disorders, including psoriasis, a common and debilitating T-cell-driven disorder characterized by thick erythematous skin plaques. ACKR2 expression is abnormal in psoriatic skin, with decreased expression correlating with recruitment of T-cells into the epidermis and increased inflammation. However, the molecular mechanisms that govern ACKR2 expression are not known. Here, we identified specific psoriasis-associated microRNAs (miRs) that bind ACKR2, inhibit its expression, and are active in primary cultures of human cutaneous cells. Using both in silico and in vitro approaches, we show that miR-146b and miR-10b directly bind the ACKR2 3'-UTR and reduce expression of ACKR2 transcripts and protein in keratinocytes and lymphatic endothelial cells, respectively. Moreover, we demonstrate that ACKR2 expression is further down-regulated upon cell trauma, an important trigger for the development of new plaques in many psoriasis patients (the Koebner phenomenon). We found that tensile cell stress leads to rapid ACKR2 down-regulation and concurrent miR-146b up-regulation. Together, we provide, for the first time, evidence for epigenetic regulation of an atypical chemokine receptor. We propose a mechanism by which cell trauma and miRs coordinately exacerbate inflammation via down-regulation of ACKR2 expression and provide a putative mechanistic explanation for the Koebner phenomenon in psoriasis.
Keywords: chemokine; immunology; inflammation; microRNA (miRNA); psoriasis.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
Spread of Psoriasiform Inflammation to Remote Tissues Is Restricted by the Atypical Chemokine Receptor ACKR2.J Invest Dermatol. 2017 Jan;137(1):85-94. doi: 10.1016/j.jid.2016.07.039. Epub 2016 Aug 26. J Invest Dermatol. 2017. PMID: 27568525 Free PMC article.
-
MiRNA expression in psoriatic skin: reciprocal regulation of hsa-miR-99a and IGF-1R.PLoS One. 2011;6(6):e20916. doi: 10.1371/journal.pone.0020916. Epub 2011 Jun 7. PLoS One. 2011. PMID: 21687694 Free PMC article.
-
SERPINB2 and miR-146a/b are coordinately regulated and act in the suppression of psoriasis-associated inflammatory responses in keratinocytes.Exp Dermatol. 2020 Jan;29(1):51-60. doi: 10.1111/exd.14049. Epub 2019 Nov 13. Exp Dermatol. 2020. PMID: 31630447
-
Koebner phenomenon leading to the formation of new psoriatic lesions: evidences and mechanisms.Biosci Rep. 2019 Dec 20;39(12):BSR20193266. doi: 10.1042/BSR20193266. Biosci Rep. 2019. PMID: 31710084 Free PMC article. Review.
-
ACKR2: Nature's Decoy Receptor Lures Unsuspecting Chemokines in Psoriasis.J Invest Dermatol. 2017 Jan;137(1):7-11. doi: 10.1016/j.jid.2016.09.035. J Invest Dermatol. 2017. PMID: 28010760
Cited by
-
Selected miRNA and Psoriasis-Cardiovascular Disease (CVD)-Overweight/Obesity Network-A Pilot Study.Int J Mol Sci. 2023 Sep 10;24(18):13916. doi: 10.3390/ijms241813916. Int J Mol Sci. 2023. PMID: 37762217 Free PMC article.
-
The Role of Atypical Chemokine Receptor D6 (ACKR2) in Physiological and Pathological Conditions; Friend, Foe, or Both?Front Immunol. 2022 May 23;13:861931. doi: 10.3389/fimmu.2022.861931. eCollection 2022. Front Immunol. 2022. PMID: 35677043 Free PMC article. Review.
-
Atypical chemokine receptor 2 expression is directly regulated by hypoxia inducible factor-1 alpha in cancer cells under hypoxia.Sci Rep. 2024 Nov 4;14(1):26589. doi: 10.1038/s41598-024-77628-8. Sci Rep. 2024. PMID: 39496762 Free PMC article.
-
Understanding psoriasis: Role of miRNAs.Biomed Rep. 2018 Nov;9(5):367-374. doi: 10.3892/br.2018.1146. Epub 2018 Sep 11. Biomed Rep. 2018. PMID: 30402223 Free PMC article. Review.
-
Advances in Understanding the Immunological Pathways in Psoriasis.Int J Mol Sci. 2019 Feb 10;20(3):739. doi: 10.3390/ijms20030739. Int J Mol Sci. 2019. PMID: 30744173 Free PMC article. Review.
References
-
- Bachelerie F., Ben-Baruch A., Burkhardt A. M., Combadiere C., Farber J. M., Graham G. J., Horuk R., Sparre-Ulrich A. H., Locati M., Luster A. D., Mantovani A., Matsushima K., Murphy P. M., Nibbs R., Nomiyama H., et al. (2014) International Union of Pharmacology. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol. Rev. 66, 1–79 10.1124/pr.113.007724 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

