Fiber-Mediated Nourishment of Gut Microbiota Protects against Diet-Induced Obesity by Restoring IL-22-Mediated Colonic Health
- PMID: 29276170
- PMCID: PMC6005180
- DOI: 10.1016/j.chom.2017.11.003
Fiber-Mediated Nourishment of Gut Microbiota Protects against Diet-Induced Obesity by Restoring IL-22-Mediated Colonic Health
Abstract
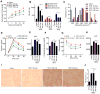
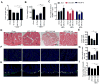
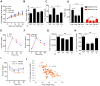
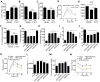
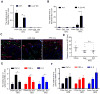
Dietary supplementation with fermentable fiber suppresses adiposity and the associated parameters of metabolic syndrome. Microbiota-generated fiber-derived short-chain fatty acids (SCFAs) and free fatty acid receptors including GPR43 are thought to mediate these effects. We find that while fermentable (inulin), but not insoluble (cellulose), fiber markedly protected mice against high-fat diet (HFD)-induced metabolic syndrome, the effect was not significantly impaired by either inhibiting SCFA production or genetic ablation of GPR43. Rather, HFD decimates gut microbiota, resulting in loss of enterocyte proliferation, leading to microbiota encroachment, low-grade inflammation (LGI), and metabolic syndrome. Enriching HFD with inulin restored microbiota loads, interleukin-22 (IL-22) production, enterocyte proliferation, and antimicrobial gene expression in a microbiota-dependent manner, as assessed by antibiotic and germ-free approaches. Inulin-induced IL-22 expression, which required innate lymphoid cells, prevented microbiota encroachment and protected against LGI and metabolic syndrome. Thus, fermentable fiber protects against metabolic syndrome by nourishing microbiota to restore IL-22-mediated enterocyte function.
Keywords: germ-free mice; intestinal inflammation; metabolic syndrome; microbiota encroachment; short-chain fatty acids.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

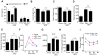
Comment in
-
Gut microbiota: Fibre restores healthy gut microbiota.Nat Rev Endocrinol. 2018 Feb;14(2):63. doi: 10.1038/nrendo.2017.182. Epub 2017 Dec 29. Nat Rev Endocrinol. 2018. PMID: 29286051 No abstract available.
-
Gut microbiota: Filling up on fibre for a healthy gut.Nat Rev Gastroenterol Hepatol. 2018 Feb;15(2):67. doi: 10.1038/nrgastro.2018.2. Epub 2018 Jan 17. Nat Rev Gastroenterol Hepatol. 2018. PMID: 29339808 No abstract available.
Similar articles
-
Inulin Fermentable Fiber Ameliorates Type I Diabetes via IL22 and Short-Chain Fatty Acids in Experimental Models.Cell Mol Gastroenterol Hepatol. 2021;12(3):983-1000. doi: 10.1016/j.jcmgh.2021.04.014. Epub 2021 Apr 30. Cell Mol Gastroenterol Hepatol. 2021. PMID: 33940221 Free PMC article.
-
Effects of dietary inulin on bacterial growth, short-chain fatty acid production and hepatic lipid metabolism in gnotobiotic mice.J Nutr Biochem. 2015 Sep;26(9):929-37. doi: 10.1016/j.jnutbio.2015.03.010. Epub 2015 May 1. J Nutr Biochem. 2015. PMID: 26033744
-
Lack of soluble fiber drives diet-induced adiposity in mice.Am J Physiol Gastrointest Liver Physiol. 2015 Oct 1;309(7):G528-41. doi: 10.1152/ajpgi.00172.2015. Epub 2015 Jul 16. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 26185332 Free PMC article.
-
Molecular link between dietary fibre, gut microbiota and health.Mol Biol Rep. 2020 Aug;47(8):6229-6237. doi: 10.1007/s11033-020-05611-3. Epub 2020 Jul 4. Mol Biol Rep. 2020. PMID: 32623619 Review.
-
Short-chain free-fatty acid G protein-coupled receptors in colon cancer.Biochem Pharmacol. 2021 Apr;186:114483. doi: 10.1016/j.bcp.2021.114483. Epub 2021 Feb 23. Biochem Pharmacol. 2021. PMID: 33631190 Review.
Cited by
-
Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids.Cell Mol Immunol. 2021 May;18(5):1161-1171. doi: 10.1038/s41423-020-00625-0. Epub 2021 Apr 13. Cell Mol Immunol. 2021. PMID: 33850311 Free PMC article. Review.
-
Circulating Short-Chain Fatty Acids Are Positively Associated with Adiposity Measures in Chinese Adults.Nutrients. 2020 Jul 17;12(7):2127. doi: 10.3390/nu12072127. Nutrients. 2020. PMID: 32708978 Free PMC article.
-
Inulin Fermentable Fiber Ameliorates Type I Diabetes via IL22 and Short-Chain Fatty Acids in Experimental Models.Cell Mol Gastroenterol Hepatol. 2021;12(3):983-1000. doi: 10.1016/j.jcmgh.2021.04.014. Epub 2021 Apr 30. Cell Mol Gastroenterol Hepatol. 2021. PMID: 33940221 Free PMC article.
-
Fiber-enriched botanicals: A therapeutic tool against certain metabolic ailments.Food Sci Nutr. 2022 Aug 26;10(10):3203-3218. doi: 10.1002/fsn3.2920. eCollection 2022 Oct. Food Sci Nutr. 2022. PMID: 36249968 Free PMC article. Review.
-
Effect of diet on pathogen performance in the microbiome.Microbiome Res Rep. 2022 Mar 26;1(2):13. doi: 10.20517/mrr.2021.10. eCollection 2022. Microbiome Res Rep. 2022. PMID: 38045644 Free PMC article. Review.
References
-
- Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

