Generic wound signals initiate regeneration in missing-tissue contexts
- PMID: 29273738
- PMCID: PMC5741630
- DOI: 10.1038/s41467-017-02338-x
Generic wound signals initiate regeneration in missing-tissue contexts
Abstract
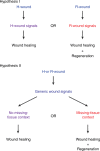
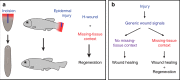
Despite the identification of numerous regulators of regeneration in different animal models, a fundamental question remains: why do some wounds trigger the full regeneration of lost body parts, whereas others resolve by mere healing? By selectively inhibiting regeneration initiation, but not the formation of a wound epidermis, here we create headless planarians and finless zebrafish. Strikingly, in both missing-tissue contexts, injuries that normally do not trigger regeneration activate complete restoration of heads and fin rays. Our results demonstrate that generic wound signals have regeneration-inducing power. However, they are interpreted as regeneration triggers only in a permissive tissue context: when body parts are missing, or when tissue-resident polarity signals, such as Wnt activity in planarians, are modified. Hence, the ability to decode generic wound-induced signals as regeneration-initiating cues may be the crucial difference that distinguishes animals that regenerate from those that cannot.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
A diffusible signal derived from hematopoietic cells supports the survival and proliferation of regenerative cells during zebrafish fin fold regeneration.Dev Biol. 2015 Mar 1;399(1):80-90. doi: 10.1016/j.ydbio.2014.12.015. Epub 2014 Dec 19. Dev Biol. 2015. PMID: 25533245
-
Heterogeneous fates and dynamic rearrangement of regenerative epidermis-derived cells during zebrafish fin regeneration.Development. 2018 Apr 13;145(8):dev162016. doi: 10.1242/dev.162016. Development. 2018. PMID: 29615465
-
Calcineurin controls proximodistal blastema polarity in zebrafish fin regeneration.Proc Natl Acad Sci U S A. 2021 Jan 12;118(2):e2009539118. doi: 10.1073/pnas.2009539118. Proc Natl Acad Sci U S A. 2021. PMID: 33376206 Free PMC article.
-
Linking wound response and inflammation to regeneration in the zebrafish larval fin.Int J Dev Biol. 2018;62(6-7-8):473-477. doi: 10.1387/ijdb.170331hr. Int J Dev Biol. 2018. PMID: 29938759 Review.
-
Zebrafish fin and heart: what's special about regeneration?Curr Opin Genet Dev. 2016 Oct;40:48-56. doi: 10.1016/j.gde.2016.05.011. Epub 2016 Jun 25. Curr Opin Genet Dev. 2016. PMID: 27351724 Review.
Cited by
-
Scar-Free Healing of Endometrium: Tissue-Specific Program of Stromal Cells and Its Induction by Soluble Factors Produced After Damage.Front Cell Dev Biol. 2021 Feb 25;9:616893. doi: 10.3389/fcell.2021.616893. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33718358 Free PMC article.
-
LINE-1 RNA triggers matrix formation in bone cells via a PKR-mediated inflammatory response.EMBO J. 2024 Sep;43(17):3587-3603. doi: 10.1038/s44318-024-00143-z. Epub 2024 Jul 1. EMBO J. 2024. PMID: 38951609 Free PMC article.
-
DNA damage and tissue repair: What we can learn from planaria.Semin Cell Dev Biol. 2019 Mar;87:145-159. doi: 10.1016/j.semcdb.2018.04.013. Epub 2018 May 3. Semin Cell Dev Biol. 2019. PMID: 29727725 Free PMC article. Review.
-
Ca2+ Signaling and Regeneration.Cold Spring Harb Perspect Biol. 2019 Nov 1;11(11):a035485. doi: 10.1101/cshperspect.a035485. Cold Spring Harb Perspect Biol. 2019. PMID: 31308144 Free PMC article. Review.
-
Epithelial Infection With Candida albicans Elicits a Multi-System Response in Planarians.Front Microbiol. 2021 Jan 14;11:629526. doi: 10.3389/fmicb.2020.629526. eCollection 2020. Front Microbiol. 2021. PMID: 33519792 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

