Salmonella enterica serovar Choleraesuis vector delivering SaoA antigen confers protection against Streptococcus suis serotypes 2 and 7 in mice and pigs
- PMID: 29268787
- PMCID: PMC5740921
- DOI: 10.1186/s13567-017-0494-6
Salmonella enterica serovar Choleraesuis vector delivering SaoA antigen confers protection against Streptococcus suis serotypes 2 and 7 in mice and pigs
Abstract
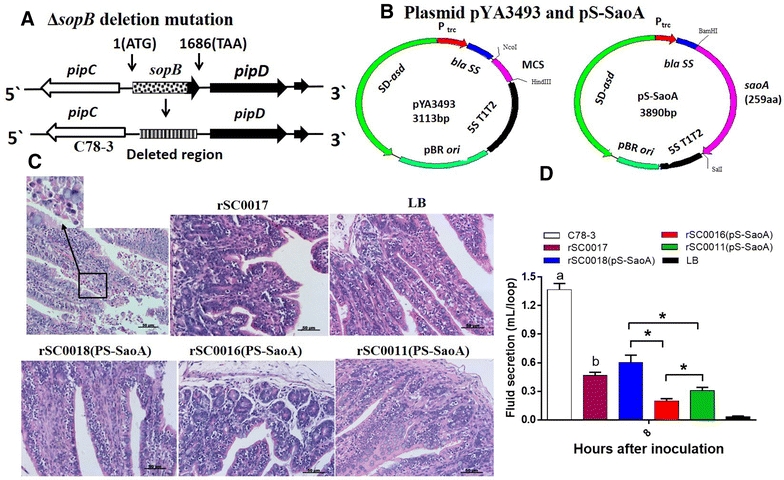
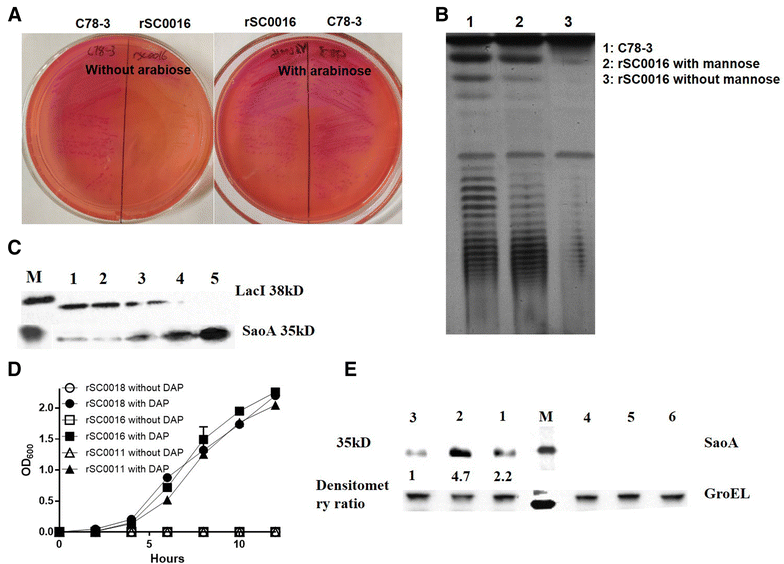
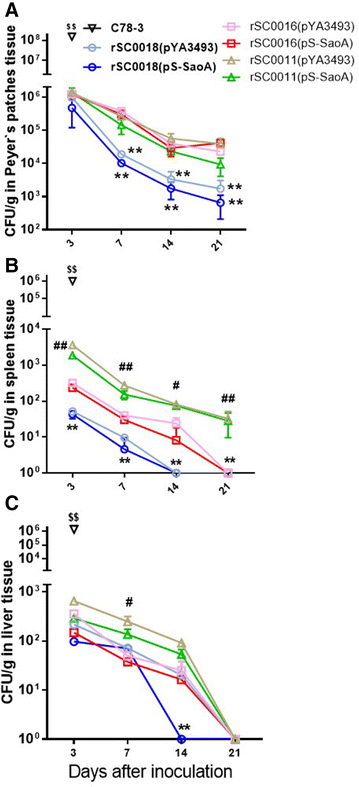
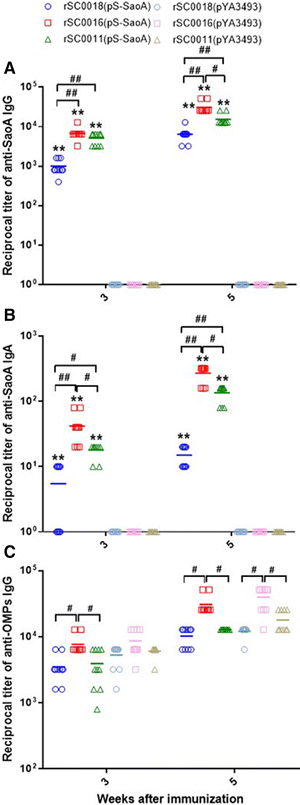
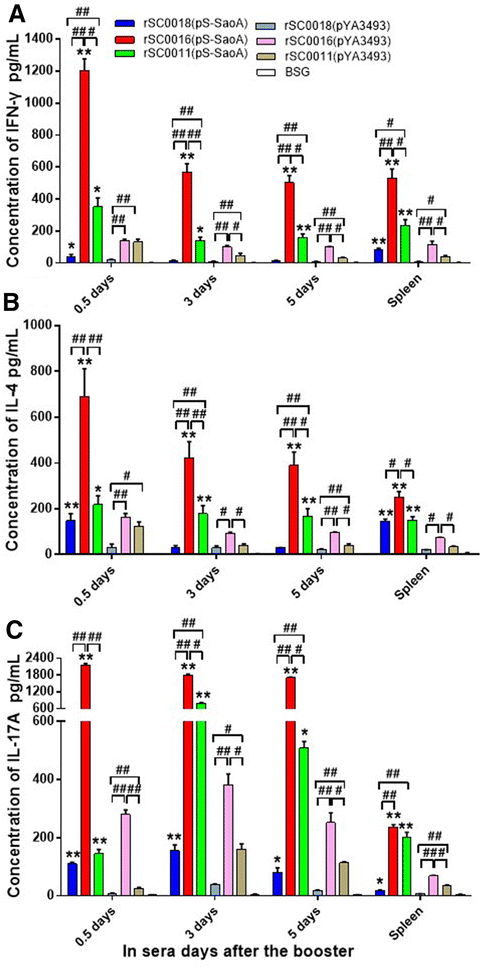
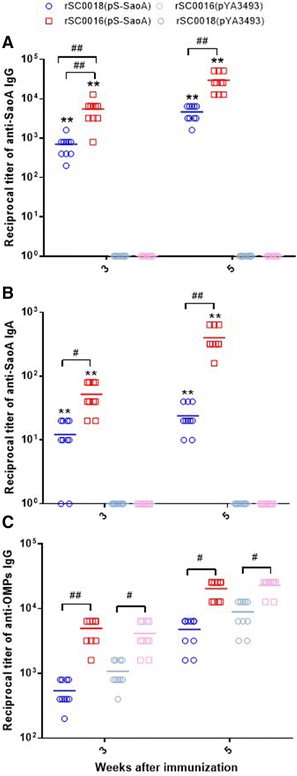
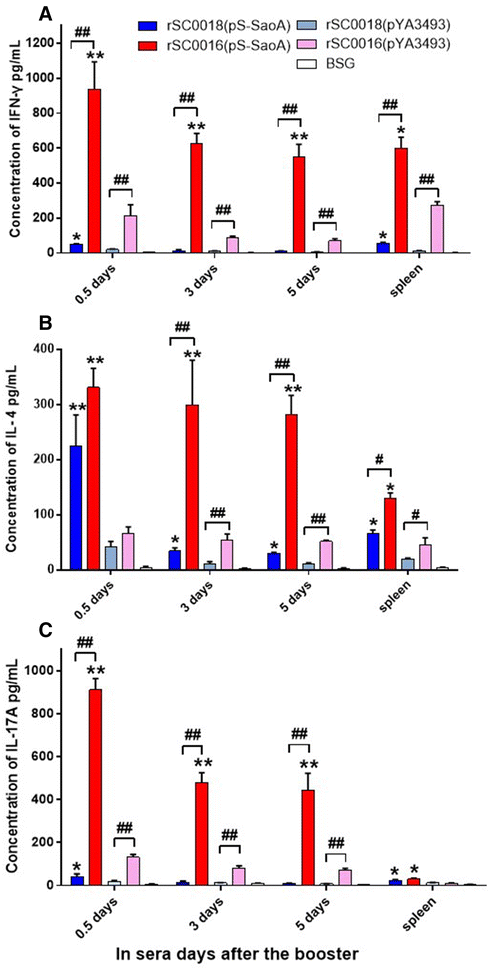
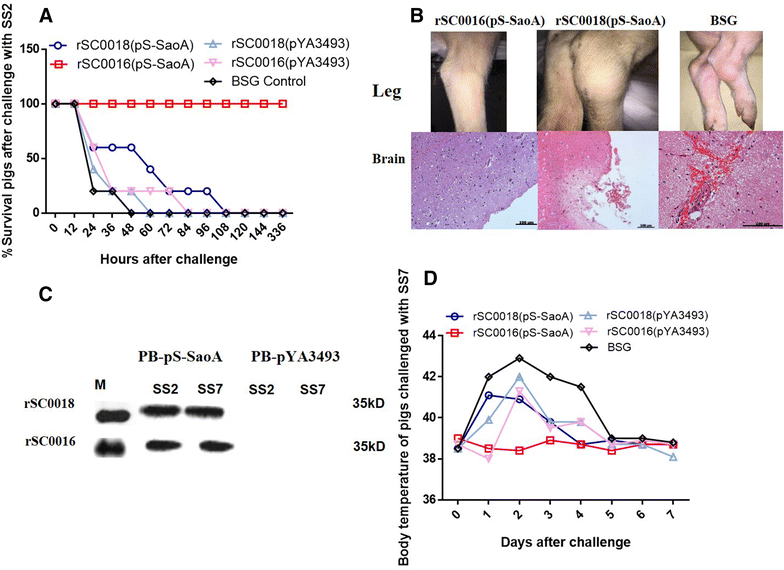
Streptococcus suis is one of the major pathogens that cause economic losses in the swine industry worldwide. However, current bacterins only provide limited prophylactic protection in the field. An ideal vaccine against S. suis should protect pigs against the clinical diseases caused by multiple serotypes, or at least protect against the dominant serotype in a given geographic region. A new recombinant Salmonella enterica serotype Choleraesuis vaccine vector, rSC0011, that is based on the regulated delayed attenuation system and regulated delayed antigen synthesis system, was developed recently. In this study, an improved recombinant attenuated Salmonella Choleraesuis vector, rSC0016, was developed by incorporating a sopB mutation to ensure adequate safety and maximal immunogenicity. In the spleens of mice, rSC0016 colonized less than rSC0011. rSC0016 and rSC0011 colonized similarly in Peyer's patches of mice. The recombinant vaccine rSC0016(pS-SaoA) induced stronger cellular, humoral, and mucosal immune responses in mice and swine against SaoA, a conserved surface protein that is present in many S. suis serotypes, than did rSC0011(pS-SaoA) without sopB or rSC0018(pS-SaoA), which is an avirulent, chemically attenuated vaccine strain. rSC0016(pS-SaoA) provided 100% protection against S. suis serotype 2 in mice and pigs, and full cross-protection against SS7 in pigs. This new vaccine vector provides a foundation for the development of a universal vaccine against multiple serotypes of S. suis in pigs.
Figures
Similar articles
-
Live-attenuated Salmonella enterica serotype Choleraesuis vaccine with regulated delayed fur mutation confer protection against Streptococcus suis in mice.BMC Vet Res. 2020 May 7;16(1):129. doi: 10.1186/s12917-020-02340-4. BMC Vet Res. 2020. PMID: 32381017 Free PMC article.
-
Live attenuated Salmonella enterica serovar Choleraesuis vaccine vector displaying regulated delayed attenuation and regulated delayed antigen synthesis to confer protection against Streptococcus suis in mice.Vaccine. 2015 Sep 11;33(38):4858-67. doi: 10.1016/j.vaccine.2015.07.063. Epub 2015 Aug 1. Vaccine. 2015. PMID: 26238722
-
Live attenuated Salmonella enterica serovar Choleraesuis vector delivering a conserved surface protein enolase induces high and broad protection against Streptococcus suis serotypes 2, 7, and 9 in mice.Vaccine. 2020 Oct 14;38(44):6904-6913. doi: 10.1016/j.vaccine.2020.08.062. Epub 2020 Sep 6. Vaccine. 2020. PMID: 32907758
-
Streptococcus suis vaccines: candidate antigens and progress.Expert Rev Vaccines. 2015;14(12):1587-608. doi: 10.1586/14760584.2015.1101349. Epub 2015 Oct 15. Expert Rev Vaccines. 2015. PMID: 26468755 Review.
-
Surface-associated and secreted factors of Streptococcus suis in epidemiology, pathogenesis and vaccine development.Anim Health Res Rev. 2009 Jun;10(1):65-83. doi: 10.1017/S146625230999003X. Anim Health Res Rev. 2009. PMID: 19558750 Review.
Cited by
-
Engineered Bacterial Outer Membrane Vesicles with Lipidated Heterologous Antigen as an Adjuvant-Free Vaccine Platform for Streptococcus suis.Appl Environ Microbiol. 2023 Mar 29;89(3):e0204722. doi: 10.1128/aem.02047-22. Epub 2023 Feb 21. Appl Environ Microbiol. 2023. PMID: 36809058 Free PMC article.
-
Live-attenuated Salmonella enterica serotype Choleraesuis vaccine with regulated delayed fur mutation confer protection against Streptococcus suis in mice.BMC Vet Res. 2020 May 7;16(1):129. doi: 10.1186/s12917-020-02340-4. BMC Vet Res. 2020. PMID: 32381017 Free PMC article.
-
Immunogenicity and protective efficacy of a Streptococcus suis vaccine composed of six conserved immunogens.Vet Res. 2021 Aug 25;52(1):112. doi: 10.1186/s13567-021-00981-3. Vet Res. 2021. PMID: 34433500 Free PMC article.
-
The role of TolA, TolB, and TolR in cell morphology, OMVs production, and virulence of Salmonella Choleraesuis.AMB Express. 2022 Jan 25;12(1):5. doi: 10.1186/s13568-022-01347-4. AMB Express. 2022. PMID: 35075554 Free PMC article.
-
Salmonella enterica serovar Choleraesuis vector delivering a dual-antigen expression cassette provides mouse cross-protection against Streptococcus suis serotypes 2, 7, 9, and 1/2.Vet Res. 2022 Jun 22;53(1):46. doi: 10.1186/s13567-022-01062-9. Vet Res. 2022. PMID: 35733156 Free PMC article.
References
-
- Segura M, Zheng H, de Greeff A, Gao GF, Grenier D, Jiang Y, Lu C, Maskell D, Oishi K, Okura M, Osawa R, Schultsz C, Schwerk C, Sekizaki T, Smith H, Srimanote P, Takamatsu D, Tang J, Tenenbaum T, Tharavichitkul P, Hoa NT, Valentin-Weigand P, Wells JM, Wertheim H, Zhu B, Xu J, Gottschalk M. Latest developments on Streptococcus suis: an emerging zoonotic pathogen: part 2. Future Microbiol. 2014;9:587–591. doi: 10.2217/fmb.14.15. - DOI - PubMed
-
- Segura M, Zheng H, de Greeff A, Gao GF, Grenier D, Jiang Y, Lu C, Maskell D, Oishi K, Okura M, Osawa R, Schultsz C, Schwerk C, Sekizaki T, Smith H, Srimanote P, Takamatsu D, Tang J, Tenenbaum T, Tharavichitkul P, Hoa NT, Valentin-Weigand P, Wells JM, Wertheim H, Zhu B, Gottschalk M, Xu J. Latest developments on Streptococcus suis: an emerging zoonotic pathogen: part 1. Future Microbiol. 2014;9:441–444. doi: 10.2217/fmb.14.14. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

