Using immunocompromised mice to identify mechanisms of Zika virus transmission and pathogenesis
- PMID: 29266213
- PMCID: PMC5838421
- DOI: 10.1111/imm.12883
Using immunocompromised mice to identify mechanisms of Zika virus transmission and pathogenesis
Abstract
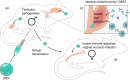
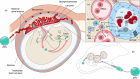
Zika virus (ZIKV) is responsible for a recent global epidemic that has been associated with congenital brain malformations in fetuses and with Guillain-Barré syndrome in adults. Within the last 2 years, a major effort has been made to develop murine models to study the mechanism of viral transmission, pathogenesis and the host immune response. Here, we discuss the findings from these models regarding the role that the innate and adaptive immune responses have in controlling ZIKV infection and pathogenesis. Additionally, we examine how innate and adaptive immune responses influence sexual and vertical transmission of ZIKV infection as well as how these responses can influence the ability of ZIKV to cross the placenta and to induce damage in the developing brain.
Keywords: T cell; innate receptors; neuroinflammation; reproductive Immunology; viral.
Published 2017. This article is a U.S. Government work and is in the public domain in the USA.
Figures
Similar articles
-
Zika Virus Infects Early- and Midgestation Human Maternal Decidual Tissues, Inducing Distinct Innate Tissue Responses in the Maternal-Fetal Interface.J Virol. 2017 Jan 31;91(4):e01905-16. doi: 10.1128/JVI.01905-16. Print 2017 Feb 15. J Virol. 2017. PMID: 27974560 Free PMC article.
-
Zika Virus Attenuation by Codon Pair Deoptimization Induces Sterilizing Immunity in Mouse Models.J Virol. 2018 Aug 16;92(17):e00701-18. doi: 10.1128/JVI.00701-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29925661 Free PMC article.
-
Recombinant Chimpanzee Adenovirus Vaccine AdC7-M/E Protects against Zika Virus Infection and Testis Damage.J Virol. 2018 Feb 26;92(6):e01722-17. doi: 10.1128/JVI.01722-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29298885 Free PMC article.
-
Pathogenesis and Molecular Mechanisms of Zika Virus.Semin Reprod Med. 2016 Sep;34(5):266-272. doi: 10.1055/s-0036-1592071. Epub 2016 Sep 9. Semin Reprod Med. 2016. PMID: 27612156 Review.
-
Hide and Seek: The Interplay Between Zika Virus and the Host Immune Response.Front Immunol. 2021 Oct 21;12:750365. doi: 10.3389/fimmu.2021.750365. eCollection 2021. Front Immunol. 2021. PMID: 34745123 Free PMC article. Review.
Cited by
-
Three Immunocompetent Small Animal Models That Do Not Support Zika Virus Infection.Pathogens. 2021 Jul 30;10(8):971. doi: 10.3390/pathogens10080971. Pathogens. 2021. PMID: 34451435 Free PMC article.
-
Mapping innate and adaptive immune function in arbovirus infections.Immunology. 2018 May;154(1):1-2. doi: 10.1111/imm.12932. Immunology. 2018. PMID: 29667753 Free PMC article. No abstract available.
-
Placental Myeloid Cells Protect against Zika Virus Vertical Transmission in a Rag1-Deficient Mouse Model.J Immunol. 2020 Jul 1;205(1):143-152. doi: 10.4049/jimmunol.1901289. Epub 2020 Jun 3. J Immunol. 2020. PMID: 32493813 Free PMC article.
-
Host genetic control of mosquito-borne Flavivirus infections.Mamm Genome. 2018 Aug;29(7-8):384-407. doi: 10.1007/s00335-018-9775-2. Epub 2018 Aug 25. Mamm Genome. 2018. PMID: 30167843 Free PMC article. Review.
-
Genetic Diversity of Collaborative Cross Mice Controls Viral Replication, Clinical Severity, and Brain Pathology Induced by Zika Virus Infection, Independently of Oas1b.J Virol. 2020 Jan 17;94(3):e01034-19. doi: 10.1128/JVI.01034-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31694939 Free PMC article.
References
-
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. N Engl J Med 2016; 374:1552–63. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

