Rapid in Vitro Quantification of S. aureus Biofilms on Vascular Graft Surfaces
- PMID: 29259580
- PMCID: PMC5723318
- DOI: 10.3389/fmicb.2017.02333
Rapid in Vitro Quantification of S. aureus Biofilms on Vascular Graft Surfaces
Abstract
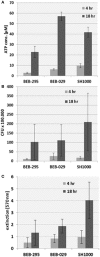
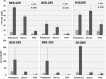
Objectives: Increasing resistance of microorganisms and particularly tolerance of bacterial biofilms against antibiotics require the need for alternative antimicrobial substances. S. aureus is the most frequent pathogen causing vascular graft infections. In order to evaluate the antimicrobial efficacy, quantification of the bacterial biofilms is necessary. Aim of the present study was the validation of an in vitro model for quantification of bacterial biofilm on vascular graft surfaces using three different assays. Methods: Standardized discs of vascular graft material (Dacron or PTFE) or polystyrene (PS) as control surface with 0.25 cm2 surface area were inoculated with 10-3 diluted overnight culture of three biofilm-producing S. aureus isolates (BEB-029, BEB-295, SH1000) in 96-well PS culture plates. After incubation for 4 and 18 h, the biofilm was determined by three different methods: (a) mitochondrial ATP concentration as measure of bacterial viability (ATP), (b) crystal violet staining (Cry), and (c) vital cell count by calculation of colony-forming units (CFU). The experiments were performed three times. Quadruplicates were used for each isolate, time point, and method. In parallel, bacterial biofilms were documented via scanning electron microscopy. Results: All three methods could quantify biofilms on the PS control. Time needed was 0:40, 13:10, and 14:30 h for ATP, Cry, and CFU, respectively. The Cry assay could not be used for vascular graft surfaces due to high unspecific background staining. However, ATP assay and CFU count showed comparable results on vascular graft material and control. The correlations between ATP and CFU assay differed according to the surface and incubation time and were significant only after 4 h on Dacron (BEB-029, p = 0.013) and on PS (BEB-029, p < 0.001). Between ATP and Cry assay on PS, a significant correlation could be detected after 4 h (BEB-295, p = 0.027) and after 18 h (all three strains, p < 0.026). The reproducibility of the ATP-assay presented as inter-assay-variance of 2.1 and as intra-assay variance of 8.1 on polystyrene. Conclusion: The in-vitro model reproducibly quantifies biofilm on standardized vascular graft surfaces with ATP assay as detection system. The ATP assay allows accelerated microbial quantification, however the correlation with the CFU assay may be strain- and surface-dependent.
Keywords: ATP assay; antimicrobial activity; biofilm quantification; colony-forming units (CFU); crystal violet staining (Cry); vascular graft.
Figures


Similar articles
-
In vitro development of Staphylococcus aureus biofilms using slime-producing variants and ATP-bioluminescence for automated bacterial quantification.Luminescence. 1999 Jan-Feb;14(1):23-31. doi: 10.1002/(SICI)1522-7243(199901/02)14:1<23::AID-BIO513>3.0.CO;2-M. Luminescence. 1999. PMID: 10398557
-
Quantifying implant-associated biofilms: Comparison of microscopic, microbiologic and biochemical methods.J Microbiol Methods. 2016 Nov;130:61-68. doi: 10.1016/j.mimet.2016.07.016. Epub 2016 Jul 19. J Microbiol Methods. 2016. PMID: 27444546
-
Biofilm Formation of Staphylococcus aureus on Various Surfaces and Their Resistance to Chlorine Sanitizer.J Food Sci. 2015 Oct;80(10):M2279-86. doi: 10.1111/1750-3841.13017. Epub 2015 Sep 28. J Food Sci. 2015. PMID: 26417663
-
Antimicrobial Blue Light Inactivation of Polymicrobial Biofilms.Front Microbiol. 2019 Apr 9;10:721. doi: 10.3389/fmicb.2019.00721. eCollection 2019. Front Microbiol. 2019. PMID: 31024499 Free PMC article.
-
A Narrative Review of Experimental Assessment to Study Vascular Biomaterials Infections and Infectability.EJVES Vasc Forum. 2023 May 12;59:49-55. doi: 10.1016/j.ejvsvf.2023.05.002. eCollection 2023. EJVES Vasc Forum. 2023. PMID: 37408851 Free PMC article. Review.
Cited by
-
Bacterial Biofilm Growth on 3D-Printed Materials.Front Microbiol. 2021 May 28;12:646303. doi: 10.3389/fmicb.2021.646303. eCollection 2021. Front Microbiol. 2021. PMID: 34122361 Free PMC article.
-
Methicillin-Susceptible Staphylococcus aureus Biofilm Formation on Vascular Grafts: an In Vitro Study.Microbiol Spectr. 2023 Feb 7;11(2):e0393122. doi: 10.1128/spectrum.03931-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36749062 Free PMC article.
-
Quantitative Examination of Antibiotic Susceptibility of Neisseria gonorrhoeae Aggregates Using ATP-utilization Commercial Assays and Live/Dead Staining.J Vis Exp. 2019 Feb 8;(144):10.3791/58978. doi: 10.3791/58978. J Vis Exp. 2019. PMID: 30799857 Free PMC article.
-
Comparative in vitro activity of bacteriophage endolysin HY-133 against Staphylococcus aureus attached to vascular graft surface.Med Microbiol Immunol. 2020 Feb;209(1):51-57. doi: 10.1007/s00430-019-00638-1. Epub 2019 Oct 17. Med Microbiol Immunol. 2020. PMID: 31624909
-
Biomechanical Stability and Osteogenesis in a Tibial Bone Defect Treated by Autologous Ovine Cord Blood Cells-A Pilot Study.Molecules. 2019 Jan 15;24(2):295. doi: 10.3390/molecules24020295. Molecules. 2019. PMID: 30650584 Free PMC article.
References
-
- Becker K., Pagnier I., Schuhen B., Wenzelburger F., Friedrich A. W., Kipp F., et al. . (2006). Does nasal cocolonization by methicillin-resistant coagulase-negative staphylococci and methicillin-susceptible Staphylococcus aureus strains occur frequently enough to represent a risk of false-positive methicillin-resistant S. aureus determinations by molecular methods? J. Clin. Microbiol. 44, 229–231. 10.1128/JCM.44.1.229-231.2006 - DOI - PMC - PubMed
-
- Bisdas T., Beckmann E., Marsch G., Burgwitz K., Wilhelmi M., Kuehn C., et al. . (2012). Prevention of vascular graft infections with antibiotic graft impregnation prior to implantation: in vitro comparison between daptomycin, rifampin and nebacetin. Eur. J. Vasc. Endovasc. Surg. 43, 448–456. 10.1016/j.ejvs.2011.12.029 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

