Production of fluorescent and cytotoxic K28 killer toxin variants through high cell density fermentation of recombinant Pichia pastoris
- PMID: 29258515
- PMCID: PMC5735513
- DOI: 10.1186/s12934-017-0844-0
Production of fluorescent and cytotoxic K28 killer toxin variants through high cell density fermentation of recombinant Pichia pastoris
Abstract
Background: Virus infected killer strains of the baker's yeast Saccharomyces cerevisiae secrete protein toxins such as K28, K1, K2 and Klus which are lethal to sensitive yeast strains of the same or related species. K28 is somewhat unique as it represents an α/β heterodimeric protein of the A/B toxin family which, after having bound to the surface of sensitive target cells, is taken up by receptor-mediated endocytosis and transported through the secretory pathway in a retrograde manner. While the current knowledge on yeast killer toxins is largely based on genetic screens for yeast mutants with altered toxin sensitivity, in vivo imaging of cell surface binding and intracellular toxin transport is still largely hampered by a lack of fluorescently labelled and biologically active killer toxin variants.
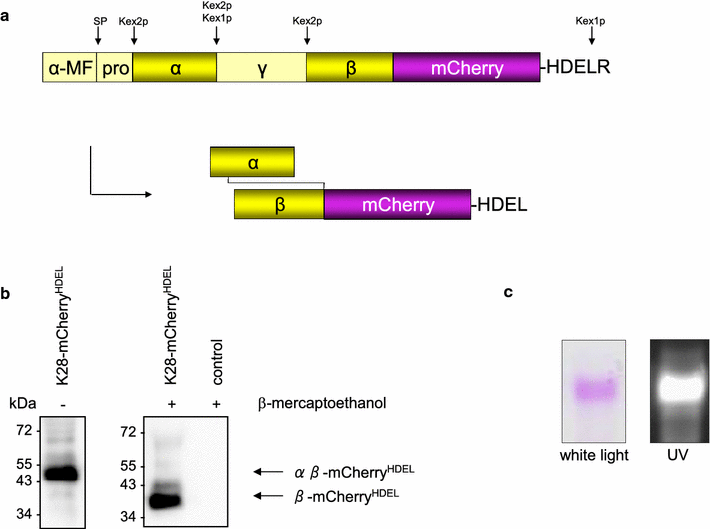
Results: In this study, we succeeded for the first time in the heterologous K28 preprotoxin expression and production of fluorescent K28 variants in Pichia pastoris. Recombinant P. pastoris GS115 cells were shown to successfully process and secrete K28 variants fused to mCherry or mTFP by high cell density fermentation. The fluorescent K28 derivatives were obtained in high yield and possessed in vivo toxicity and specificity against sensitive yeast cells. In cell binding studies the resulting K28 variants caused strong fluorescence signals at the cell periphery due to toxin binding to primary K28 receptors within the yeast cell wall. Thereby, the β-subunit of K28 was confirmed to be the sole component required and sufficient for K28 cell wall binding.
Conclusion: Successful production of fluorescent killer toxin variants of S. cerevisiae by high cell density fermentation of recombinant, K28 expressing strains of P. pastoris now opens the possibility to study and monitor killer toxin cell surface binding, in particular in toxin resistant yeast mutants in which toxin resistance is caused by defects in toxin binding due to alterations in cell wall structure and composition. This novel approach might be easily transferable to other killer toxins from different yeast species and genera. Furthermore, the fluorescent toxin variants described here might likewise represent a powerful tool in future studies to visualize intracellular A/B toxin trafficking with the help of high resolution single molecule imaging techniques.
Keywords: A/B toxins; Fluorescence labelling; Heterologous protein expression; High cell density fermentation; Killer toxin; Pichia pastoris; Saccharomyces cerevisiae.
Figures
Similar articles
-
Yeast Killer Toxin K28: Biology and Unique Strategy of Host Cell Intoxication and Killing.Toxins (Basel). 2017 Oct 20;9(10):333. doi: 10.3390/toxins9100333. Toxins (Basel). 2017. PMID: 29053588 Free PMC article. Review.
-
Viral preprotoxin signal sequence allows efficient secretion of green fluorescent protein by Candida glabrata, Pichia pastoris, Saccharomyces cerevisiae, and Schizosaccharomyces pombe.Appl Environ Microbiol. 2004 Feb;70(2):961-6. doi: 10.1128/AEM.70.2.961-966.2004. Appl Environ Microbiol. 2004. PMID: 14766577 Free PMC article.
-
A yeast killer toxin screen provides insights into a/b toxin entry, trafficking, and killing mechanisms.Dev Cell. 2009 Oct;17(4):552-60. doi: 10.1016/j.devcel.2009.08.006. Dev Cell. 2009. PMID: 19853568 Free PMC article.
-
Relationships and Evolution of Double-Stranded RNA Totiviruses of Yeasts Inferred from Analysis of L-A-2 and L-BC Variants in Wine Yeast Strain Populations.Appl Environ Microbiol. 2017 Feb 1;83(4):e02991-16. doi: 10.1128/AEM.02991-16. Print 2017 Feb 15. Appl Environ Microbiol. 2017. PMID: 27940540 Free PMC article.
-
The Biology of Pichia membranifaciens Killer Toxins.Toxins (Basel). 2017 Mar 23;9(4):112. doi: 10.3390/toxins9040112. Toxins (Basel). 2017. PMID: 28333108 Free PMC article. Review.
Cited by
-
Use of red, far-red, and near-infrared light in imaging of yeasts and filamentous fungi.Appl Microbiol Biotechnol. 2022 Jun;106(11):3895-3912. doi: 10.1007/s00253-022-11967-2. Epub 2022 May 23. Appl Microbiol Biotechnol. 2022. PMID: 35599256 Free PMC article. Review.
-
Advanced Situation with Recombinant Toxins: Diversity, Production and Application Purposes.Int J Mol Sci. 2023 Feb 27;24(5):4630. doi: 10.3390/ijms24054630. Int J Mol Sci. 2023. PMID: 36902061 Free PMC article. Review.
-
Production of Industrial Enzymes via Pichia pastoris as a Cell Factory in Bioreactor: Current Status and Future Aspects.Protein J. 2021 Jun;40(3):367-376. doi: 10.1007/s10930-021-09968-7. Epub 2021 Feb 15. Protein J. 2021. PMID: 33587243 Review.
References
-
- Bevan EA, Makower M. The physiological basis of the killer character in yeast. Proc Xlth Int Congr Genet. 1963;1:202–203.
-
- Rodriguez-Cousino N, Maqueda M, Ambrona J, Zamora E, Esteban R, Ramirez M. A new wine Saccharomyces cerevisiae killer toxin (Klus), encoded by a double-stranded rna virus, with broad antifungal activity is evolutionarily related to a chromosomal host gene. Appl Environ Microbiol. 2011;77(5):1822–1832. doi: 10.1128/AEM.02501-10. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

