Organ-specific lymphatic vasculature: From development to pathophysiology
- PMID: 29242199
- PMCID: PMC5748863
- DOI: 10.1084/jem.20171868
Organ-specific lymphatic vasculature: From development to pathophysiology
Abstract
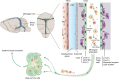
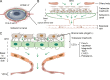
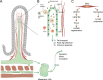
Recent discoveries of novel functions and diverse origins of lymphatic vessels have drastically changed our view of lymphatic vasculature. Traditionally regarded as passive conduits for fluid and immune cells, lymphatic vessels now emerge as active, tissue-specific players in major physiological and pathophysiological processes. Lymphatic vessels show remarkable plasticity and heterogeneity, reflecting their functional specialization to control the tissue microenvironment. Moreover, alternative developmental origins of lymphatic endothelial cells in some organs may contribute to the diversity of their functions in adult tissues. This review aims to summarize the most recent findings of organotypic differentiation of lymphatic endothelial cells in terms of their distinct (patho)physiological functions in skin, lymph nodes, small intestine, brain, and eye. We discuss recent advances in our understanding of the heterogeneity of lymphatic vessels with respect to the organ-specific functional and molecular specialization of lymphatic endothelium, such as the hybrid blood-lymphatic identity of Schlemm's canal, functions of intestinal lymphatics in dietary fat uptake, and discovery of meningeal lymphatic vasculature and perivascular brain lymphatic endothelial cells.
© 2018 Petrova and Koh.
Figures


Similar articles
-
Heterogeneity in the lymphatic vascular system and its origin.Cardiovasc Res. 2016 Sep;111(4):310-21. doi: 10.1093/cvr/cvw175. Epub 2016 Jun 29. Cardiovasc Res. 2016. PMID: 27357637 Free PMC article. Review.
-
Lymphatics in nanophysiology.Adv Drug Deliv Rev. 2014 Jul;74:12-8. doi: 10.1016/j.addr.2014.01.011. Epub 2014 Feb 10. Adv Drug Deliv Rev. 2014. PMID: 24524932 Review.
-
When form meets function: the cells and signals that shape the lymphatic vasculature during development.Development. 2021 Jun 1;148(11):dev167098. doi: 10.1242/dev.167098. Epub 2021 Jun 3. Development. 2021. PMID: 34080610 Review.
-
The lymphatic vasculature revisited.J Clin Invest. 2014 Mar;124(3):874-7. doi: 10.1172/JCI74854. Epub 2014 Mar 3. J Clin Invest. 2014. PMID: 24590271 Free PMC article.
-
DLL4 promotes continuous adult intestinal lacteal regeneration and dietary fat transport.J Clin Invest. 2015 Nov 3;125(12):4572-86. doi: 10.1172/JCI82045. J Clin Invest. 2015. PMID: 26529256 Free PMC article.
Cited by
-
Minimally Invasive Glaucoma Surgery: What Do We Know? Where Should We Go?Transl Vis Sci Technol. 2020 Apr 23;9(5):15. doi: 10.1167/tvst.9.5.15. eCollection 2020 Apr. Transl Vis Sci Technol. 2020. PMID: 32821487 Free PMC article. Review.
-
Cross‑talk between lymphangiogenesis and malignant melanoma cells: New opinions on tumour drainage and immunization (Review).Oncol Lett. 2024 Jan 5;27(2):81. doi: 10.3892/ol.2024.14215. eCollection 2024 Feb. Oncol Lett. 2024. PMID: 38249813 Free PMC article. Review.
-
Dermal Microvascular Units in Domestic Pigs (Sus scrofa domestica): Role as Transdermal Passive Immune Channels.Front Vet Sci. 2022 Apr 25;9:891286. doi: 10.3389/fvets.2022.891286. eCollection 2022. Front Vet Sci. 2022. PMID: 35548054 Free PMC article.
-
Therapeutic Regeneration of Lymphatic and Immune Cell Functions upon Lympho-organoid Transplantation.Stem Cell Reports. 2019 Jun 11;12(6):1260-1268. doi: 10.1016/j.stemcr.2019.04.021. Epub 2019 May 30. Stem Cell Reports. 2019. PMID: 31155505 Free PMC article.
-
The Glymphatic System: A Novel Component of Fundamental Neurobiology.J Neurosci. 2021 Sep 15;41(37):7698-7711. doi: 10.1523/JNEUROSCI.0619-21.2021. J Neurosci. 2021. PMID: 34526407 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

