Increased single-strand annealing rather than non-homologous end-joining predicts hereditary ovarian carcinoma
- PMID: 29228718
- PMCID: PMC5716758
- DOI: 10.18632/oncotarget.21720
Increased single-strand annealing rather than non-homologous end-joining predicts hereditary ovarian carcinoma
Abstract
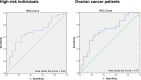
Mutations in genes encoding DNA double-strand break (DSB) repair components, especially homologous recombination (HR) proteins, were found to predispose to breast and ovarian cancer. Beyond high penetrance risk gene mutations underlying monogenic defects, low risk gene mutations generate polygenic defects, enlarging the fraction of individuals with a predisposing phenotype. DSB repair dysfunction opens new options for targeted therapies; poly (ADP-ribose) polymerase (PARP) inhibitors have been approved for BRCA-mutated and platinum-responsive ovarian cancers. In this work, we performed functional analyses in peripheral blood lymphocytes (PBLs) using a case-control design. We examined 38 women with familial history of breast and/or ovarian cancer, 40 women with primary ovarian cancer and 34 healthy controls. Using a GFP-based test we analyzed error-prone DSB repair mechanisms which are known to compensate for HR defects and to generate chromosomal instabilities. While non-homologous end-joining (NHEJ) did not discriminate between cases and controls, we found increases of single-strand annealing (SSA) in women with familial risk vs. controls (P=0.016) and patients with ovarian cancer vs. controls (P=0.002). Consistent with compromised HR we also detected increased sensitivities to carboplatin in PBLs from high-risk individuals (P<0.0001) as well as patients (P=0.0011) compared to controls. Conversely, neither PARP inhibitor responses nor PARP activities were altered in PBLs from the case groups, but PARP activities increased with age in high-risk individuals, providing novel clues for differential drug mode-of-action. Our findings indicate the great potential of detecting SSA activities to deliver an estimate of ovarian cancer susceptibility and therapeutic responsiveness beyond the limitations of genotyping.
Keywords: PARP activity; early-onset ovarian cancer; error-prone DNA repair; functional biomarker; ovarian cancer risk.
Conflict of interest statement
CONFLICTS OF INTEREST None of the authors has any conflicts of interest. L.W. is an inventor of a patent on a test system for determining genotoxicities, which is owned by L.W.
Figures
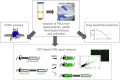
 initiated NHEJ deleting the spacer sequence (grey bar) between the promoter (kinked arrow) and GFP coding region (green bar) in EJ5SceGFP. Cleavage within internally mutated HR-EGFP triggered homologous repair with N-terminally mutated 3´EGFP in substrate HR-EGFP/3´EGFP. Remaining PBLs were subjected to the analysis of drug sensitivities using MTT assay, whereby IC50-values were determined after carboplatin and PARP inhibitor (IQD) treatment.
initiated NHEJ deleting the spacer sequence (grey bar) between the promoter (kinked arrow) and GFP coding region (green bar) in EJ5SceGFP. Cleavage within internally mutated HR-EGFP triggered homologous repair with N-terminally mutated 3´EGFP in substrate HR-EGFP/3´EGFP. Remaining PBLs were subjected to the analysis of drug sensitivities using MTT assay, whereby IC50-values were determined after carboplatin and PARP inhibitor (IQD) treatment.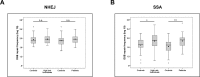


Similar articles
-
The power of DNA double-strand break (DSB) repair testing to predict breast cancer susceptibility.FASEB J. 2012 May;26(5):2094-104. doi: 10.1096/fj.11-200790. Epub 2012 Jan 25. FASEB J. 2012. PMID: 22278937
-
Age-related activity of Poly (ADP-Ribose) Polymerase (PARP) in men with localized prostate cancer.Mech Ageing Dev. 2021 Jun;196:111494. doi: 10.1016/j.mad.2021.111494. Epub 2021 Apr 19. Mech Ageing Dev. 2021. PMID: 33887280
-
In vitro model for DNA double-strand break repair analysis in breast cancer reveals cell type-specific associations with age and prognosis.FASEB J. 2016 Nov;30(11):3786-3799. doi: 10.1096/fj.201600453R. Epub 2016 Aug 5. FASEB J. 2016. PMID: 27494941
-
Therapeutic targeting and patient selection for cancers with homologous recombination defects.Expert Opin Drug Discov. 2017 Jun;12(6):565-581. doi: 10.1080/17460441.2017.1322061. Epub 2017 May 2. Expert Opin Drug Discov. 2017. PMID: 28425306 Review.
-
Harnessing DNA Double-Strand Break Repair for Cancer Treatment.Front Oncol. 2019 Dec 10;9:1388. doi: 10.3389/fonc.2019.01388. eCollection 2019. Front Oncol. 2019. PMID: 31921645 Free PMC article. Review.
Cited by
-
Genetic modifiers regulating DNA replication and double-strand break repair are associated with differences in mammary tumors in mouse models of Li-Fraumeni syndrome.Oncogene. 2021 Aug;40(31):5026-5037. doi: 10.1038/s41388-021-01892-5. Epub 2021 Jun 28. Oncogene. 2021. PMID: 34183771 Free PMC article.
-
Combinations of ATR, Chk1 and Wee1 Inhibitors with Olaparib Are Active in Olaparib Resistant Brca1 Proficient and Deficient Murine Ovarian Cells.Cancers (Basel). 2022 Apr 1;14(7):1807. doi: 10.3390/cancers14071807. Cancers (Basel). 2022. PMID: 35406579 Free PMC article.
-
Biallelic germline BRCA1 mutations in a patient with early onset breast cancer, mild Fanconi anemia-like phenotype, and no chromosome fragility.Mol Genet Genomic Med. 2019 Sep;7(9):e863. doi: 10.1002/mgg3.863. Epub 2019 Jul 25. Mol Genet Genomic Med. 2019. PMID: 31347298 Free PMC article.
-
Sex-specific differences in DNA double-strand break repair of cycling human lymphocytes during aging.Aging (Albany NY). 2021 Sep 10;13(17):21066-21089. doi: 10.18632/aging.203519. Epub 2021 Sep 10. Aging (Albany NY). 2021. PMID: 34506302 Free PMC article.
-
Single-Strand Annealing in Cancer.Int J Mol Sci. 2021 Feb 22;22(4):2167. doi: 10.3390/ijms22042167. Int J Mol Sci. 2021. PMID: 33671579 Free PMC article. Review.
References
-
- Stratton JF, Pharoah P, Smith SK, Easton D, Ponder BA. A systematic review and meta-analysis of family history and risk of ovarian cancer. Br J Obstet Gynaecol. 1998;105:493–499. - PubMed
-
- Kleibl Z, Kristensen VN. Women at high risk of breast cancer: Molecular characteristics, clinical presentation and management. Breast. 2016;28:136–144. - PubMed
-
- Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, Roeb W, Agnew KJ, Stray SM, Wickramanayake A, Norquist B, Pennington KP, Garcia RL, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A. 2011;108:18032–18037. - PMC - PubMed
-
- Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, Thornton A, Norquist BM, Casadei S, Nord AS, Agnew KJ, Pritchard CC, Scroggins S, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764–775. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

