Estimating Cost-effectiveness of a Multimodal Ovarian Cancer Screening Program in the United States: Secondary Analysis of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS)
- PMID: 29222541
- PMCID: PMC5838595
- DOI: 10.1001/jamaoncol.2017.4211
Estimating Cost-effectiveness of a Multimodal Ovarian Cancer Screening Program in the United States: Secondary Analysis of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS)
Abstract
Importance: The United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) is the largest randomized clinical trial to evaluate screening's impact on ovarian cancer mortality, assigning women to multimodal screening (MMS) with serum cancer antigen 125 (CA-125) interpreted using a risk algorithm. If the MMS screening method is eventually shown to reduce mortality and be cost-effective, then it may be accepted by the medical community as a feasible screening tool.
Objective: To estimate the cost-effectiveness of an MMS screening program in the United States.
Design, setting, and participants: A Markov simulation model was constructed using data from UKCTOCS to compare MMS with no screening in the United States. Screening would begin at the age of 50 years for women in the general population. Published estimates of the long-term effect of MMS screening on ovarian cancer mortality and the trial's published hazard ratios were used to simulate mortality estimates up to 40 years from start of screening. Base-case costs included CA-125, ultrasound, and false-positive work-up results, in addition to a risk algorithm cost estimate of $100. The utility and costs of ovarian cancer treatment were incorporated into the model.
Interventions: Screening strategies varied by costs of the algorithm and treatment for advanced ovarian cancer, rates of screening compliance, ovarian cancer incidence, and extrapolation of ovarian cancer mortality.
Main outcomes and measures: Costs, quality-adjusted life-years (QALYs), and mortality reduction of ovarian cancer screening.
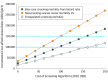
Results: Multimodal screening is both more expensive and more effective in reducing ovarian cancer mortality over a lifetime than no screening. After accounting for uncertainty in the underlying parameters, screening women starting at age 50 years with MMS is cost-effective 70% of the time, when decision makers are willing to pay $150 000 per QALY. Screening reduced mortality by 15%, with an incremental cost-effectiveness ratio (ICER) ranging from $106 187 (95% CI, $97 496-$127 793) to $155 256 (95% CI, $150 369-$198 567).
Conclusions and relevance: Ovarian cancer screening is potentially cost-effective in the United States depending on final significance of mortality reduction and cost of the CA-125 risk algorithm. These results are limited by uncertainty around the effect of screening on ovarian cancer mortality beyond the 11 years of UKCTOCS.
Conflict of interest statement
Figures
Similar articles
-
The cost-effectiveness of screening for ovarian cancer: results from the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS).Br J Cancer. 2017 Aug 22;117(5):619-627. doi: 10.1038/bjc.2017.222. Epub 2017 Jul 25. Br J Cancer. 2017. PMID: 28742794 Free PMC article.
-
Cost-effectiveness of screening for ovarian cancer amongst postmenopausal women: a model-based economic evaluation.BMC Med. 2016 Dec 6;14(1):200. doi: 10.1186/s12916-016-0743-y. BMC Med. 2016. PMID: 27919292 Free PMC article.
-
Mortality impact, risks, and benefits of general population screening for ovarian cancer: the UKCTOCS randomised controlled trial.Health Technol Assess. 2023 May 11:1-81. doi: 10.3310/BHBR5832. Online ahead of print. Health Technol Assess. 2023. PMID: 37183782 Free PMC article.
-
Screening for Ovarian Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force.JAMA. 2018 Feb 13;319(6):595-606. doi: 10.1001/jama.2017.21421. JAMA. 2018. PMID: 29450530 Review.
-
Screening for Ovarian Cancer: An Updated Evidence Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Feb. Report No.: 17-05231-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Feb. Report No.: 17-05231-EF-1. PMID: 29648765 Free Books & Documents. Review.
Cited by
-
Liquid biopsy in ovarian cancer in China and the world: current status and future perspectives.Front Oncol. 2023 Dec 19;13:1276085. doi: 10.3389/fonc.2023.1276085. eCollection 2023. Front Oncol. 2023. PMID: 38169730 Free PMC article. Review.
-
ANXA2P2: A Potential Immunological and Prognostic Signature in Ovarian Serous Cystadenocarcinoma via Pan-Carcinoma Synthesis.Front Oncol. 2022 Feb 8;12:818977. doi: 10.3389/fonc.2022.818977. eCollection 2022. Front Oncol. 2022. PMID: 35211410 Free PMC article.
-
Identification of SAA1 as a novel metastasis marker in ovarian cancer and development of a graphene-based detection platform for early assessment.J Cancer Res Clin Oncol. 2023 Dec;149(18):16391-16406. doi: 10.1007/s00432-023-05296-8. Epub 2023 Sep 14. J Cancer Res Clin Oncol. 2023. PMID: 37707574
-
The cuproptosis-related gene signature serves as a potential prognostic predictor for ovarian cancer using bioinformatics analysis.Ann Transl Med. 2022 Sep;10(18):1021. doi: 10.21037/atm-22-4546. Ann Transl Med. 2022. PMID: 36267774 Free PMC article.
-
Hereditary Ovarian Cancer: Towards a Cost-Effective Prevention Strategy.Int J Environ Res Public Health. 2022 Sep 23;19(19):12057. doi: 10.3390/ijerph191912057. Int J Environ Res Public Health. 2022. PMID: 36231355 Free PMC article. Review.
References
-
- Are W. The Key Statistics About Ovarian Cancer? Cancer A-Z 2017; https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html. Accessed April 5, 2017.
-
- van Nagell JR Jr, DePriest PD, Ueland FR, et al. . Ovarian cancer screening with annual transvaginal sonography: findings of 25,000 women screened. Cancer. 2007;109(9):1887-1896. - PubMed
-
- Jacobs IJ, Skates SJ, MacDonald N, et al. . Screening for ovarian cancer: a pilot randomised controlled trial. Lancet. 1999;353(9160):1207-1210. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

