Pioglitazone Inhibits Periprostatic White Adipose Tissue Inflammation in Obese Mice
- PMID: 29222347
- PMCID: PMC5882568
- DOI: 10.1158/1940-6207.CAPR-17-0296
Pioglitazone Inhibits Periprostatic White Adipose Tissue Inflammation in Obese Mice
Abstract
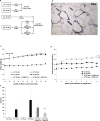
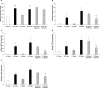
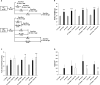
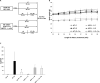
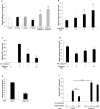
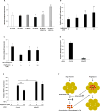
Obesity is associated with an increased incidence of high-grade prostate cancer and poor prognosis for prostate cancer patients. Recently, we showed that obesity-related periprostatic white adipose tissue (WAT) inflammation, characterized by crown-like structures (CLS) consisting of dead or dying adipocytes surrounded by macrophages, was associated with high-grade prostate cancer. It is possible, therefore, that agents that suppress periprostatic WAT inflammation will alter the development or progression of prostate cancer. Pioglitazone, a ligand of PPARγ, is used to treat diabetes and possesses anti-inflammatory properties. Here, our main objectives were to determine whether pioglitazone inhibited obesity-related periprostatic WAT inflammation in mice and then to elucidate the underlying mechanism. Treatment with pioglitazone reduced the density of CLS in periprostatic fat and suppressed levels of TNFα, TGFβ, and the chemokine monocyte chemoattractant protein-1 (MCP-1). Importantly, the ability of pioglitazone to suppress periprostatic WAT inflammation was abrogated in MCP-1 knockout mice. Pioglitazone caused dose-dependent induction of both adiponectin, an anti-inflammatory adipokine, and its receptor AdipoR2 in cultured 3T3-L1 cells and in periprostatic WAT of obese mice. Pioglitazone blocked TNFα-mediated induction of MCP-1 in 3T3-L1 cells, an effect that was attenuated when either adiponectin or AdipoR2 were silenced. Taken together, pioglitazone-mediated induction of adiponectin suppressed the elevation in MCP-1 levels, thereby attenuating obesity-related periprostatic WAT inflammation. These findings strengthen the rationale for future efforts to determine whether targeting the PPARγ-adiponectin-MCP-1 axis will decrease periprostatic adipose inflammation and thereby reduce the risk of high-grade prostate cancer or improve outcomes for men with prostate cancer. Cancer Prev Res; 11(4); 215-26. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures
Similar articles
-
Supplemental estrogen and caloric restriction reduce obesity-induced periprostatic white adipose inflammation in mice.Carcinogenesis. 2019 Jul 20;40(7):914-923. doi: 10.1093/carcin/bgz088. Carcinogenesis. 2019. PMID: 31067318 Free PMC article.
-
Periprostatic adipose inflammation is associated with high-grade prostate cancer.Prostate Cancer Prostatic Dis. 2017 Dec;20(4):418-423. doi: 10.1038/pcan.2017.31. Epub 2017 Jun 27. Prostate Cancer Prostatic Dis. 2017. PMID: 28653675 Free PMC article.
-
Effect of pioglitazone on metabolic features in endotoxemia model in obese diabetic db/db mice.J Diabetes. 2017 Jun;9(6):613-621. doi: 10.1111/1753-0407.12450. Epub 2016 Aug 17. J Diabetes. 2017. PMID: 27530729
-
Omega-3 fatty acids and adipose tissue biology.Mol Aspects Med. 2018 Dec;64:147-160. doi: 10.1016/j.mam.2018.01.004. Epub 2018 Jan 17. Mol Aspects Med. 2018. PMID: 29329795 Review.
-
Preventive and improvement effects of exercise training and supplement intake in white adipose tissues on obesity and lifestyle-related diseases.Environ Health Prev Med. 2012 Sep;17(5):348-56. doi: 10.1007/s12199-012-0271-0. Epub 2012 Feb 24. Environ Health Prev Med. 2012. PMID: 22362099 Free PMC article. Review.
Cited by
-
Repurposing of Chronically Used Drugs in Cancer Therapy: A Chance to Grasp.Cancers (Basel). 2023 Jun 15;15(12):3199. doi: 10.3390/cancers15123199. Cancers (Basel). 2023. PMID: 37370809 Free PMC article. Review.
-
Promising drug repurposing approach targeted for cytokine storm implicated in SARS-CoV-2 complications.Immunopharmacol Immunotoxicol. 2021 Aug;43(4):395-409. doi: 10.1080/08923973.2021.1931302. Epub 2021 May 31. Immunopharmacol Immunotoxicol. 2021. PMID: 34057871 Free PMC article. Review.
-
Obesity and Breast Cancer: The Role of Crown-Like Structures in Breast Adipose Tissue in Tumor Progression, Prognosis, and Therapy.J Breast Cancer. 2020 Jun;23(3):233-245. doi: 10.4048/jbc.2020.23.e35. J Breast Cancer. 2020. PMID: 32595986 Free PMC article. Review.
-
A gut microbial metabolite of linoleic acid ameliorates liver fibrosis by inhibiting TGF-β signaling in hepatic stellate cells.Sci Rep. 2023 Nov 3;13(1):18983. doi: 10.1038/s41598-023-46404-5. Sci Rep. 2023. PMID: 37923895 Free PMC article.
-
Adipose tissue inflammation linked to obesity: A review of current understanding, therapies and relevance of phyto-therapeutics.Heliyon. 2023 Dec 2;10(1):e23114. doi: 10.1016/j.heliyon.2023.e23114. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38163110 Free PMC article. Review.
References
-
- Hammarsten J, Hogstedt B. Hyperinsulinaemia: a prospective risk factor for lethal clinical prostate cancer. Eur J Cancer. 2005;41(18):2887–95. doi S0959-8049(05)00765-3 [pii] 10.1016/j.ejca.2005.09.003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

