Fission yeast Ccq1 is a modulator of telomerase activity
- PMID: 29216371
- PMCID: PMC5778466
- DOI: 10.1093/nar/gkx1223
Fission yeast Ccq1 is a modulator of telomerase activity
Abstract
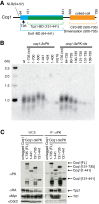
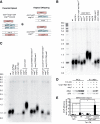
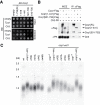
Shelterin, the telomeric protein complex, plays a crucial role in telomere homeostasis. In fission yeast, telomerase is recruited to chromosome ends by the shelterin component Tpz1 and its binding partner Ccq1, where telomerase binds to the 3' overhang to add telomeric repeats. Recruitment is initiated by the interaction of Ccq1 with the telomerase subunit Est1. However, how telomerase is released following elongation remains to be established. Here, we show that Ccq1 also has a role in the suppression of telomere elongation, when coupled with the Clr4 histone H3 methyl-transferase complex and the Clr3 histone deacetylase and nucleosome remodelling complex, SHREC. We have dissected the functions of Ccq1 by establishing a Ccq1-Est1 fusion system, which bypasses the telomerase recruitment step. We demonstrate that Ccq1 forms two distinct complexes for positive and negative telomerase regulation, with Est1 and Clr3 respectively. The negative form of Ccq1 promotes dissociation of Ccq1-telomerase from Tpz1, thereby restricting local telomerase activity. The Clr4 complex also has a negative regulation activity with Ccq1, independently of SHREC. Thus, we propose a model in which Ccq1-Est1 recruits telomerase to mediate telomere extension, whilst elongated telomeric DNA recruits Ccq1 with the chromatin-remodelling complexes, which in turn releases telomerase from the telomere.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



Similar articles
-
How long does telomerase extend telomeres? Regulation of telomerase release and telomere length homeostasis.Curr Genet. 2018 Dec;64(6):1177-1181. doi: 10.1007/s00294-018-0836-6. Epub 2018 Apr 16. Curr Genet. 2018. PMID: 29663033 Free PMC article. Review.
-
Tpz1-Ccq1 and Tpz1-Poz1 interactions within fission yeast shelterin modulate Ccq1 Thr93 phosphorylation and telomerase recruitment.PLoS Genet. 2014 Oct 16;10(10):e1004708. doi: 10.1371/journal.pgen.1004708. eCollection 2014 Oct. PLoS Genet. 2014. PMID: 25330395 Free PMC article.
-
Ccq1-Tpz1TPP1 interaction facilitates telomerase and SHREC association with telomeres in fission yeast.Mol Biol Cell. 2015 Nov 1;26(21):3857-66. doi: 10.1091/mbc.E15-07-0481. Epub 2015 Sep 9. Mol Biol Cell. 2015. PMID: 26354422 Free PMC article.
-
Roles of heterochromatin and telomere proteins in regulation of fission yeast telomere recombination and telomerase recruitment.J Biol Chem. 2010 Feb 19;285(8):5327-37. doi: 10.1074/jbc.M109.078840. Epub 2009 Dec 29. J Biol Chem. 2010. PMID: 20040595 Free PMC article.
-
Finding the end: recruitment of telomerase to telomeres.Nat Rev Mol Cell Biol. 2013 Feb;14(2):69-82. doi: 10.1038/nrm3505. Epub 2013 Jan 9. Nat Rev Mol Cell Biol. 2013. PMID: 23299958 Free PMC article. Review.
Cited by
-
RNA-DNA Hybrids Support Recombination-Based Telomere Maintenance in Fission Yeast.Genetics. 2019 Oct;213(2):431-447. doi: 10.1534/genetics.119.302606. Epub 2019 Aug 12. Genetics. 2019. PMID: 31405990 Free PMC article.
-
How long does telomerase extend telomeres? Regulation of telomerase release and telomere length homeostasis.Curr Genet. 2018 Dec;64(6):1177-1181. doi: 10.1007/s00294-018-0836-6. Epub 2018 Apr 16. Curr Genet. 2018. PMID: 29663033 Free PMC article. Review.
-
Mechanism of MRX inhibition by Rif2 at telomeres.Nat Commun. 2021 May 12;12(1):2763. doi: 10.1038/s41467-021-23035-w. Nat Commun. 2021. PMID: 33980827 Free PMC article.
-
Structural insights into Pot1-ssDNA, Pot1-Tpz1 and Tpz1-Ccq1 Interactions within fission yeast shelterin complex.PLoS Genet. 2022 Jul 18;18(7):e1010308. doi: 10.1371/journal.pgen.1010308. eCollection 2022 Jul. PLoS Genet. 2022. PMID: 35849625 Free PMC article.
-
Ccq1-Raf2 interaction mediates CLRC recruitment to establish heterochromatin at telomeres.Life Sci Alliance. 2021 Sep 7;4(11):e202101106. doi: 10.26508/lsa.202101106. Print 2021 Nov. Life Sci Alliance. 2021. PMID: 34493579 Free PMC article.
References
-
- Dehe P.M., Rog O., Ferreira M.G., Greenwood J., Cooper J.P.. Taz1 enforces cell-cycle regulation of telomere synthesis. Mol. Cell. 2012; 46:797–808. - PubMed
-
- Hector R.E., Shtofman R.L., Ray A., Chen B.R., Nyun T., Berkner K.L., Runge K.W.. Tel1p preferentially associates with short telomeres to stimulate their elongation. Mol. Cell. 2007; 27:851–858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

