Pannexin 1 Modulates Axonal Growth in Mouse Peripheral Nerves
- PMID: 29213230
- PMCID: PMC5702652
- DOI: 10.3389/fncel.2017.00365
Pannexin 1 Modulates Axonal Growth in Mouse Peripheral Nerves
Abstract
The pannexin family of channels consists of three members-pannexin-1 (Panx1), pannexin-2 (Panx2), and pannexin-3 (Panx3) that enable the exchange of metabolites and signaling molecules between intracellular and extracellular compartments. Pannexin-mediated release of intracellular ATP into the extracellular space has been tied to a number of cellular activities, primarily through the activity of type P2 purinergic receptors. Previous work indicates that the opening of Panx1 channels and activation of purinergic receptors by extracellular ATP may cause inflammation and apoptosis. In the CNS (central nervous system) and PNS (peripheral nervous system), coupled pannexin, and P2 functions have been linked to peripheral sensitization (pain) pathways. Purinergic pathways are also essential for other critical processes in the PNS, including myelination and neurite outgrowth. However, whether such pathways are pannexin-dependent remains to be determined. In this study, we use a Panx1 knockout mouse model and pharmacological inhibitors of the Panx1 and the ATP-mediated signaling pathway to fill gaps in our understanding of Panx1 localization in peripheral nerves, roles for Panx1 in axonal outgrowth and myelination, and neurite extension. Our data show that Panx1 is localized to axonal, myelin, and vascular compartments of the peripheral nerves. Knockout of Panx1 gene significantly increased axonal caliber in vivo and axonal growth rate in cultured dorsal root ganglia (DRG) neurons. Furthermore, genetic knockout of Panx1 or inhibition of components of purinergic signaling, by treatment with probenecid and apyrase, resulted in denser axonal outgrowth from cultured DRG explants compared to untreated wild-types. Our findings suggest that Panx1 regulates axonal growth in the peripheral nervous system.
Keywords: axon; dorsal root ganglion; pannexin; peripheral nerve; purinergic signaling.
Figures
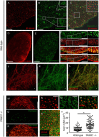
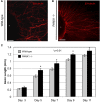
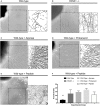
Similar articles
-
Neuronal Panx1 drives peripheral sensitization in experimental plantar inflammatory pain.Mil Med Res. 2024 Apr 29;11(1):27. doi: 10.1186/s40779-024-00525-8. Mil Med Res. 2024. PMID: 38685116 Free PMC article.
-
Pannexin 1 and pannexin 3 differentially regulate the cancer cell properties of cutaneous squamous cell carcinoma.J Physiol. 2024 Nov 19. doi: 10.1113/JP286172. Online ahead of print. J Physiol. 2024. PMID: 39560179
-
ATP stimulates pannexin 1 internalization to endosomal compartments.Biochem J. 2015 Sep 15;470(3):319-30. doi: 10.1042/BJ20141551. Epub 2015 Jul 20. Biochem J. 2015. PMID: 26195825
-
Pannexin-1 channels in epilepsy.Neurosci Lett. 2019 Mar 16;695:71-75. doi: 10.1016/j.neulet.2017.09.004. Epub 2017 Sep 5. Neurosci Lett. 2019. PMID: 28886985 Review.
-
The biochemistry and function of pannexin channels.Biochim Biophys Acta. 2013 Jan;1828(1):15-22. doi: 10.1016/j.bbamem.2012.01.017. Epub 2012 Jan 28. Biochim Biophys Acta. 2013. PMID: 22305965 Review.
Cited by
-
Acute Pannexin 1 Blockade Mitigates Early Synaptic Plasticity Defects in a Mouse Model of Alzheimer's Disease.Front Cell Neurosci. 2020 Mar 19;14:46. doi: 10.3389/fncel.2020.00046. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32265655 Free PMC article.
-
Neuronal Panx1 drives peripheral sensitization in experimental plantar inflammatory pain.Mil Med Res. 2024 Apr 29;11(1):27. doi: 10.1186/s40779-024-00525-8. Mil Med Res. 2024. PMID: 38685116 Free PMC article.
-
Mechanism of P2X7 receptor-dependent enhancement of neuromuscular transmission in pannexin 1 knockout mice.Purinergic Signal. 2018 Dec;14(4):459-469. doi: 10.1007/s11302-018-9630-7. Epub 2018 Oct 25. Purinergic Signal. 2018. PMID: 30362043 Free PMC article.
-
Postnatal Restriction of Activity-Induced Ca2+ Responses to Schwann Cells at the Neuromuscular Junction Are Caused by the Proximo-Distal Loss of Axonal Synaptic Vesicles during Development.J Neurosci. 2018 Oct 3;38(40):8650-8665. doi: 10.1523/JNEUROSCI.0956-18.2018. Epub 2018 Aug 24. J Neurosci. 2018. PMID: 30143570 Free PMC article.
-
Probenecid Disrupts a Novel Pannexin 1-Collapsin Response Mediator Protein 2 Interaction and Increases Microtubule Stability.Front Cell Neurosci. 2018 May 11;12:124. doi: 10.3389/fncel.2018.00124. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29867357 Free PMC article.
References
-
- Beckel J. M., Argall A. J., Lim J. C., Xia J., Lu W., Coffey E. E., et al. . (2014). Mechanosensitive release of adenosine 5'-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: a mechanism for purinergic involvement in chronic strain. Glia 62, 1486–1501. 10.1002/glia.22695 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

