Effects of metamizole, MAA, and paracetamol on proliferation, apoptosis, and necrosis in the pancreatic cancer cell lines PaTu 8988 t and Panc-1
- PMID: 29208039
- PMCID: PMC5717838
- DOI: 10.1186/s40360-017-0185-y
Effects of metamizole, MAA, and paracetamol on proliferation, apoptosis, and necrosis in the pancreatic cancer cell lines PaTu 8988 t and Panc-1
Abstract
Background: Adenocarcinoma of the pancreas is one of the most aggressive cancer diseases affecting the human body. Recent research has shown the importance of the perioperative phase in disease progression. Particularly during this vulnerable phase, substances such as metamizole and paracetamol are given as general anesthetics and postoperative analgesics. Therefore, the effects of metamizole and paracetamol on tumor progression should be investigated in more detail because the extent to which these substances influence the carcinogenesis of pancreatic carcinoma is still unclear. This study analyzed the influence of metamizole and its active metabolites MAA (4-N-methyl-aminoantipyrine) and paracetamol on the proliferation, apoptosis, and necrosis of the pancreatic cancer cell lines PaTu 8988t and Panc-1 in vitro.
Methods: Cell proliferation was measured by means of the ELISA BrdU assay and the rate of apoptosis by flow cytometry using the Annexin V assay.
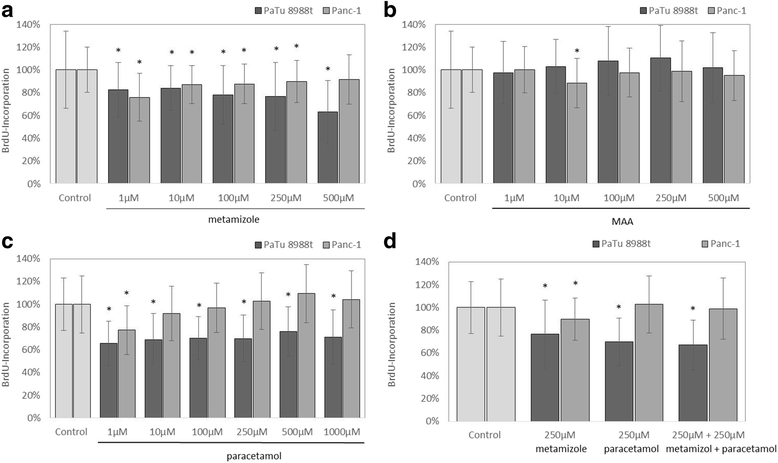
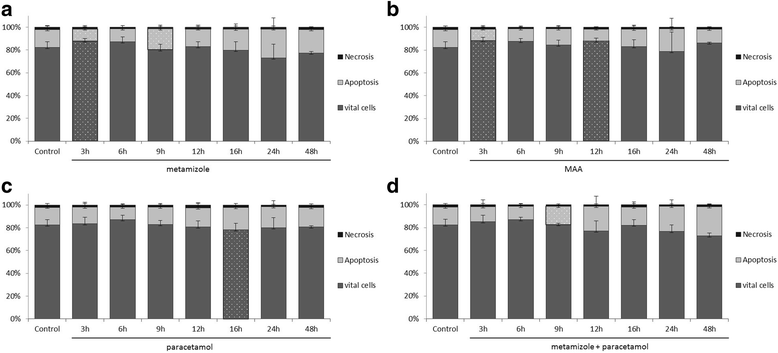
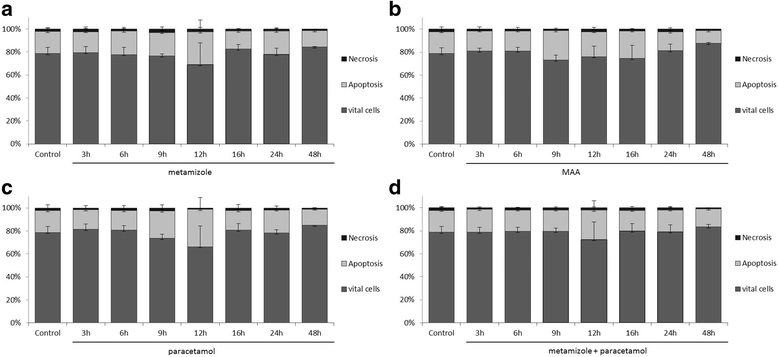
Results: Metamizole and paracetamol significantly inhibited cell proliferation in pancreatic cancer cells. After the addition of metamizole to PaTu 8988t cells, the rate of apoptosis was reduced after 3 h of incubation but significantly increased after 9 h of incubation.
Conclusion: The oncogenic potential of pancreatic adenocarcinoma is mainly characterized by its extreme growth rate. Non-opioid analgesics such as metamizole and paracetamol are given as general anesthetics and postoperative analgesics. The combination of metamizole or paracetamol with cytotoxic therapeutic approaches may achieve synergistic effects. Further studies are necessary to identify the underlying mechanisms so that new therapeutic options may be developed for the treatment of this aggressive tumor.
Keywords: Acetaminophen; Apoptosis; Cancer; Dipyrone; MAA; Metamizole; Necrosis; Pancreatic carcinoma; Paracetamol; Proliferation.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Comparison of metamizole and paracetamol effects on colonic anastomosis and fibroblast activities in Wistar rats.BMC Pharmacol Toxicol. 2020 Jan 13;21(1):6. doi: 10.1186/s40360-020-0383-x. BMC Pharmacol Toxicol. 2020. PMID: 31931882 Free PMC article.
-
Acetaminophen and Metamizole Induce Apoptosis in HT 29 and SW 480 Colon Carcinoma Cell Lines In Vitro.Anticancer Res. 2018 Feb;38(2):745-751. doi: 10.21873/anticanres.12280. Anticancer Res. 2018. PMID: 29374698
-
A Comparative Investigation of the Analgesic Effects of Metamizole and Paracetamol in Rats.J Invest Surg. 2015 Jun;28(3):173-80. doi: 10.3109/08941939.2014.998798. J Invest Surg. 2015. PMID: 26065593
-
Safety of metamizole (dipyrone) for the treatment of mild to moderate pain-an overview of systematic reviews.Naunyn Schmiedebergs Arch Pharmacol. 2024 Nov;397(11):8515-8525. doi: 10.1007/s00210-024-03240-2. Epub 2024 Jun 18. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38888755
-
[Metamizole in postoperative pain management].Ned Tijdschr Geneeskd. 2012;156(14):A4323. Ned Tijdschr Geneeskd. 2012. PMID: 22475238 Review. Dutch.
Cited by
-
Classification of clear cell renal cell carcinoma based on PKM alternative splicing.Heliyon. 2020 Feb 19;6(2):e03440. doi: 10.1016/j.heliyon.2020.e03440. eCollection 2020 Feb. Heliyon. 2020. PMID: 32095654 Free PMC article.
-
Comparison of metamizole and paracetamol effects on colonic anastomosis and fibroblast activities in Wistar rats.BMC Pharmacol Toxicol. 2020 Jan 13;21(1):6. doi: 10.1186/s40360-020-0383-x. BMC Pharmacol Toxicol. 2020. PMID: 31931882 Free PMC article.
-
Investigation of the effect of dipyrone on cells isolated from intervertebral disc tissue.Exp Ther Med. 2019 Jul;18(1):216-224. doi: 10.3892/etm.2019.7576. Epub 2019 May 13. Exp Ther Med. 2019. PMID: 31258656 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

