Ran-binding protein M is associated with human spermatogenesis and oogenesis
- PMID: 29207172
- PMCID: PMC5783472
- DOI: 10.3892/mmr.2017.8147
Ran-binding protein M is associated with human spermatogenesis and oogenesis
Abstract
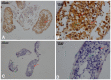
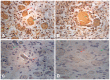
The aim of the present study was to explore the underlying mechanism and diagnostic potential of Ran‑binding protein M (RanBPM) in human spermatogenesis and oogenesis. RanBPM expression in human testis and ovaries was analysed using polymerase chain reaction (PCR) and western blotting, and immunofluorescence was performed on testis and ovary tissue sections during different developmental stages of spermatogenesis and oogenesis using RanBPM antibodies. Interactions with a variety of functional proteins were also investigated. RanBPM mRNA and protein expression levels were determined by PCR and western blotting in the tissue sections. Results revealed that the mRNA expression levels were highest in the testis followed by the ovary. The RanBPM protein was predominantly localized in the nucleus of germ cells, and the expression levels were highest in pachytene spermatocytes and cells surrounding spermatids in testis tissue. In ovary cells, RanBPM was localized in the nucleus and cytoplasm. In conclusion, the results suggested that RanBPM may have multiple roles in the regulation of germ cell proliferation during human spermatogenesis and oogenesis. This research may provide a novel insight into the underlying molecular mechanism of RanBPM and may have implications for the clinical diagnosis and treatment of human infertility.
Figures


Similar articles
-
Sperm membrane protein (hSMP-1) and RanBPM complex in the microtubule-organizing centre.J Mol Med (Berl). 2004 Jun;82(6):383-8. doi: 10.1007/s00109-004-0535-2. Epub 2004 Mar 10. J Mol Med (Berl). 2004. PMID: 15014887
-
RanBPM is essential for mouse spermatogenesis and oogenesis.Development. 2011 Jun;138(12):2511-21. doi: 10.1242/dev.062505. Epub 2011 May 11. Development. 2011. PMID: 21561988 Free PMC article.
-
RanBPM (RanBP9) regulates mouse c-Kit receptor level and is essential for normal development of bone marrow progenitor cells.Oncotarget. 2016 Dec 20;7(51):85109-85123. doi: 10.18632/oncotarget.13198. Oncotarget. 2016. PMID: 27835883 Free PMC article.
-
Diverse roles of the scaffolding protein RanBPM.Drug Discov Today. 2012 Apr;17(7-8):379-87. doi: 10.1016/j.drudis.2011.10.030. Epub 2011 Nov 7. Drug Discov Today. 2012. PMID: 22094242 Review.
-
Scaffolding protein RanBPM and its interactions in diverse signaling pathways in health and disease.Discov Med. 2018 Apr;25(138):177-194. Discov Med. 2018. PMID: 29723489 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

