Effects of Long-Term Alcohol Drinking on the Dopamine D2 Receptor: Gene Expression and Heteroreceptor Complexes in the Striatum in Rats
- PMID: 29205397
- PMCID: PMC5817245
- DOI: 10.1111/acer.13568
Effects of Long-Term Alcohol Drinking on the Dopamine D2 Receptor: Gene Expression and Heteroreceptor Complexes in the Striatum in Rats
Abstract
Background: Reduced dopamine D2 receptor (D2R) ligand binding has repeatedly been demonstrated in the striatum of humans with alcohol use disorder (AUD). The attenuated D2R binding has been suggested to reflect a reduced D2R density, which in turn has been proposed to drive craving and relapse. However, results from rodent studies addressing the effects of alcohol drinking on D2R density have been inconsistent.
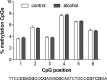
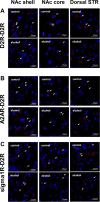
Methods: A validated alcohol drinking model (intermittent access to 20% alcohol) in Wistar rats was used to study the effects of voluntary alcohol drinking (at least 12 weeks) on the D2R in the striatum compared to age-matched alcohol-naïve control rats. Reverse transcriptase quantitative PCR was used to quantify isoform-specific Drd2 gene expression levels. Using bisulfite pyrosequencing, DNA methylation levels of a regulatory region of the Drd2 gene were determined. In situ proximity ligation assay was used to measure densities of D2R receptor complexes: D2R-D2R, adenosine A2A receptor (A2AR)-D2R, and sigma1 receptor (sigma1R)-D2R.
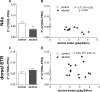
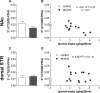
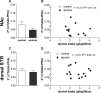
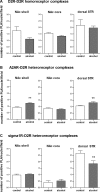
Results: Long-term voluntary alcohol drinking significantly reduced mRNA levels of the long D2R isoform in the nucleus accumbens (NAc) but did not alter CpG methylation levels in the analyzed sequence of the Drd2 gene. Alcohol drinking also reduced the striatal density of D2R-D2R homoreceptor complexes, increased the density of A2AR-D2R heteroreceptor complexes in the NAc shell and the dorsal striatum, and decreased the density of sigma1R-D2R heteroreceptor complexes in the dorsal striatum.
Conclusions: The present results on long-term alcohol drinking might reflect reduced D2R levels through reductions in D2R-D2R homoreceptor complexes and gene expression. Furthermore, based on antagonistic interactions between A2AR and D2R, an increased density of A2AR-D2R heteroreceptor complexes might indicate a reduced affinity and signaling of the D2R population within the complex. Hence, both reduced striatal D2R levels and reduced D2R protomer affinity within the striatal A2AR-D2R complex might underlie reduced D2R radioligand binding in humans with AUD. This supports the hypothesis of a hypodopaminergic system in AUD and suggests the A2AR-D2R heteroreceptor complex as a potential novel treatment target.
Keywords: Alcohol Dependence; DNA Methylation; Epigenetics; Heteroreceptors and Homoreceptors; Receptor Dimers.
Copyright © 2017 The Authors. Alcoholism: Clinical & Experimental Research published by Wiley Periodicals, Inc. on behalf of Research Society on Alcoholism.
Figures
Similar articles
-
Acute cocaine treatment enhances the antagonistic allosteric adenosine A2A-dopamine D2 receptor-receptor interactions in rat dorsal striatum without increasing significantly extracellular dopamine levels.Pharmacol Rep. 2020 Apr;72(2):332-339. doi: 10.1007/s43440-020-00069-3. Epub 2020 Mar 2. Pharmacol Rep. 2020. PMID: 32124388
-
Cocaine self-administration specifically increases A2AR-D2R and D2R-sigma1R heteroreceptor complexes in the rat nucleus accumbens shell. Relevance for cocaine use disorder.Pharmacol Biochem Behav. 2017 Apr;155:24-31. doi: 10.1016/j.pbb.2017.03.003. Epub 2017 Mar 12. Pharmacol Biochem Behav. 2017. PMID: 28300546
-
A2AR Transmembrane 2 Peptide Administration Disrupts the A2AR-A2AR Homoreceptor but Not the A2AR-D2R Heteroreceptor Complex: Lack of Actions on Rodent Cocaine Self-Administration.Int J Mol Sci. 2019 Dec 3;20(23):6100. doi: 10.3390/ijms20236100. Int J Mol Sci. 2019. PMID: 31816953 Free PMC article.
-
A2AR-D2R Heteroreceptor Complexes in Cocaine Reward and Addiction.Trends Pharmacol Sci. 2018 Dec;39(12):1008-1020. doi: 10.1016/j.tips.2018.10.007. Epub 2018 Oct 29. Trends Pharmacol Sci. 2018. PMID: 30384981 Review.
-
Understanding the Functional Plasticity in Neural Networks of the Basal Ganglia in Cocaine Use Disorder: A Role for Allosteric Receptor-Receptor Interactions in A2A-D2 Heteroreceptor Complexes.Neural Plast. 2016;2016:4827268. doi: 10.1155/2016/4827268. Epub 2016 Oct 30. Neural Plast. 2016. PMID: 27872762 Free PMC article. Review.
Cited by
-
Effects of monoamines on the intrinsic excitability of lateral orbitofrontal cortex neurons in alcohol-dependent and non-dependent female mice.Neuropharmacology. 2018 Jul 15;137:1-12. doi: 10.1016/j.neuropharm.2018.04.019. Epub 2018 Apr 22. Neuropharmacology. 2018. PMID: 29689260 Free PMC article.
-
Adolescent oxycodone exposure inhibits withdrawal-induced expression of genes associated with the dopamine transmission.Addict Biol. 2021 Jul;26(4):e12994. doi: 10.1111/adb.12994. Epub 2020 Dec 15. Addict Biol. 2021. PMID: 33325096 Free PMC article.
-
A Review of DNA Risk Alleles to Determine Epigenetic Repair of mRNA Expression to Prove Therapeutic Effectiveness in Reward Deficiency Syndrome (RDS): Embracing "Precision Behavioral Management".Psychol Res Behav Manag. 2021 Dec 17;14:2115-2134. doi: 10.2147/PRBM.S292958. eCollection 2021. Psychol Res Behav Manag. 2021. PMID: 34949945 Free PMC article. Review.
-
Increased dopaminergic neurotransmission results in ethanol dependent sedative behaviors in Caenorhabditis elegans.PLoS Genet. 2021 Feb 1;17(2):e1009346. doi: 10.1371/journal.pgen.1009346. eCollection 2021 Feb. PLoS Genet. 2021. PMID: 33524034 Free PMC article.
-
The monoamine stabilizer OSU6162 has anxiolytic-like properties and reduces voluntary alcohol intake in a genetic rat model of depression.Sci Rep. 2021 Jun 4;11(1):11856. doi: 10.1038/s41598-021-91215-1. Sci Rep. 2021. PMID: 34088937 Free PMC article.
References
-
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A (2003) Alcohol promotes dopamine release in the human nucleus accumbens. Synapse 49:226–231. - PubMed
-
- Borroto‐Escuela DO, Narvaez M, Wydra K, Pintsuk J, Pinton L, Jimenez‐Beristain A, di Palma M, Jastrzebska J, Filip M, Fuxe K (2017) Cocaine self‐administration specifically increases A2AR‐D2R and D2R‐sigma1R heteroreceptor complexes in the rat nucleus accumbens shell. Relevance for cocaine use disorder. Pharmacol Biochem Behav 155:24–31. - PubMed
-
- Borroto‐Escuela DO, Romero‐Fernandez W, Garriga P, Ciruela F, Narvaez M, Tarakanov AO, Palkovits M, Agnati LF, Fuxe K (2013) G protein‐coupled receptor heterodimerization in the brain. Methods Enzymol 521:281–294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

