The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages
- PMID: 29195811
- PMCID: PMC5773350
- DOI: 10.1016/j.immuni.2017.11.013
The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages
Abstract
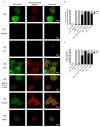
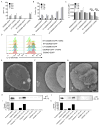
The interleukin-1 (IL-1) family cytokines are cytosolic proteins that exhibit inflammatory activity upon release into the extracellular space. These factors are released following various cell death processes, with pyroptosis being a common mechanism. Recently, it was recognized that phagocytes can achieve a state of hyperactivation, which is defined by their ability to secrete IL-1 while retaining viability, yet it is unclear how IL-1 can be secreted from living cells. Herein, we report that the pyroptosis regulator gasdermin D (GSDMD) was necessary for IL-1β secretion from living macrophages that have been exposed to inflammasome activators, such as bacteria and their products or host-derived oxidized lipids. Cell- and liposome-based assays demonstrated that GSDMD pores were required for IL-1β transport across an intact lipid bilayer. These findings identify a non-pyroptotic function for GSDMD, and raise the possibility that GSDMD pores represent conduits for the secretion of cytosolic cytokines under conditions of cell hyperactivation.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Innate immunity: Revealing the secrets of IL-1 secretion.Nat Rev Immunol. 2017 Dec 22;18(1):2-3. doi: 10.1038/nri.2017.155. Nat Rev Immunol. 2017. PMID: 29269767 No abstract available.
-
Gasdermin D Flashes an Exit Signal for IL-1.Immunity. 2018 Jan 16;48(1):1-3. doi: 10.1016/j.immuni.2018.01.003. Immunity. 2018. PMID: 29343431
Similar articles
-
Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion.Cell Res. 2015 Dec;25(12):1285-98. doi: 10.1038/cr.2015.139. Epub 2015 Nov 27. Cell Res. 2015. PMID: 26611636 Free PMC article.
-
Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death.Nature. 2015 Oct 29;526(7575):660-5. doi: 10.1038/nature15514. Epub 2015 Sep 16. Nature. 2015. PMID: 26375003
-
N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis.Nat Commun. 2020 May 5;11(1):2212. doi: 10.1038/s41467-020-16043-9. Nat Commun. 2020. PMID: 32371889 Free PMC article.
-
Uncoupled pyroptosis and IL-1β secretion downstream of inflammasome signaling.Front Immunol. 2023 Apr 6;14:1128358. doi: 10.3389/fimmu.2023.1128358. eCollection 2023. Front Immunol. 2023. PMID: 37090724 Free PMC article. Review.
-
New insights into Gasdermin D pore formation.Biochem Soc Trans. 2024 Apr 24;52(2):681-692. doi: 10.1042/BST20230549. Biochem Soc Trans. 2024. PMID: 38497302 Review.
Cited by
-
Biting the hand that feeds: Metabolic determinants of cell fate during infection.Front Immunol. 2022 Oct 13;13:923024. doi: 10.3389/fimmu.2022.923024. eCollection 2022. Front Immunol. 2022. PMID: 36311735 Free PMC article. Review.
-
Dooming Phagocyte Responses: Inflammatory Effects of Endogenous Oxidized Phospholipids.Front Endocrinol (Lausanne). 2021 Mar 15;12:626842. doi: 10.3389/fendo.2021.626842. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33790857 Free PMC article. Review.
-
NLRP3 promotes allergic responses to birch pollen extract in a model of intranasal sensitization.Front Immunol. 2024 Jun 12;15:1393819. doi: 10.3389/fimmu.2024.1393819. eCollection 2024. Front Immunol. 2024. PMID: 38933263 Free PMC article.
-
Microglial function, INPP5D/SHIP1 signaling, and NLRP3 inflammasome activation: implications for Alzheimer's disease.Mol Neurodegener. 2023 Nov 29;18(1):89. doi: 10.1186/s13024-023-00674-9. Mol Neurodegener. 2023. PMID: 38017562 Free PMC article. Review.
-
Melatonin Enhances the Therapeutic Effect of Plasma Exosomes Against Cerebral Ischemia-Induced Pyroptosis Through the TLR4/NF-κB Pathway.Front Neurosci. 2020 Aug 18;14:848. doi: 10.3389/fnins.2020.00848. eCollection 2020. Front Neurosci. 2020. PMID: 33013286 Free PMC article.
References
-
- Bera A, Herbert S, Jakob A, Vollmer W, Götz F. Why are pathogenic staphylococci so lysozyme resistant? The peptidoglycan O-acetyltransferase OatA is the major determinant for lysozyme resistance of Staphylococcus aureus. Molecular Microbiology. 2005;55:778–87. - PubMed
-
- Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA, et al. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992;256:97–100. - PubMed
-
- Chen KW, Groß CJ, Sotomayor FV, Stacey KJ, Tschopp J, Sweet MJ, Schroder K. The neutrophil NLRC4 inflammasome selectively promotes IL-1β maturation without pyroptosis during acute Salmonella challenge. Cell Reports. 2014;8(2):570–582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

