Natural Parasite Exposure Induces Protective Human Anti-Malarial Antibodies
- PMID: 29195810
- PMCID: PMC5738265
- DOI: 10.1016/j.immuni.2017.11.007
Natural Parasite Exposure Induces Protective Human Anti-Malarial Antibodies
Abstract
Antibodies against the NANP repeat of circumsporozoite protein (CSP), the major surface antigen of Plasmodium falciparum (Pf) sporozoites, can protect from malaria in animal models but protective humoral immunity is difficult to induce in humans. Here we cloned and characterized rare affinity-matured human NANP-reactive memory B cell antibodies elicited by natural Pf exposure that potently inhibited parasite transmission and development in vivo. We unveiled the molecular details of antibody binding to two distinct protective epitopes within the NANP repeat. NANP repeat recognition was largely mediated by germline encoded and immunoglobulin (Ig) heavy-chain complementarity determining region 3 (HCDR3) residues, whereas affinity maturation contributed predominantly to stabilizing the antigen-binding site conformation. Combined, our findings illustrate the power of exploring human anti-CSP antibody responses to develop tools for malaria control in the mammalian and the mosquito vector and provide a molecular basis for the structure-based design of next-generation CSP malaria vaccines.
Keywords: Plasmodium falciparum; X-ray structures; affinity maturation; circumsporozoite protein; epitope specificity; human; malaria; memory B cells; natural infection; protective antibodies.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

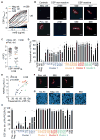

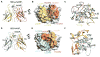
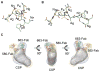
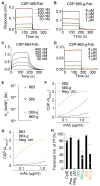
Similar articles
-
How to induce protective humoral immunity against Plasmodium falciparum circumsporozoite protein.J Exp Med. 2022 Feb 7;219(2):e20201313. doi: 10.1084/jem.20201313. Epub 2022 Jan 10. J Exp Med. 2022. PMID: 35006242 Free PMC article. Review.
-
Structural basis for antibody recognition of the NANP repeats in Plasmodium falciparum circumsporozoite protein.Proc Natl Acad Sci U S A. 2017 Nov 28;114(48):E10438-E10445. doi: 10.1073/pnas.1715812114. Epub 2017 Nov 14. Proc Natl Acad Sci U S A. 2017. PMID: 29138320 Free PMC article.
-
Diverse Antibody Responses to Conserved Structural Motifs in Plasmodium falciparum Circumsporozoite Protein.J Mol Biol. 2020 Feb 14;432(4):1048-1063. doi: 10.1016/j.jmb.2019.12.029. Epub 2019 Dec 27. J Mol Biol. 2020. PMID: 31883801 Free PMC article.
-
Avid binding by B cells to the Plasmodium circumsporozoite protein repeat suppresses responses to protective subdominant epitopes.Cell Rep. 2021 Apr 13;35(2):108996. doi: 10.1016/j.celrep.2021.108996. Cell Rep. 2021. PMID: 33852850 Free PMC article.
-
The circumsporozoite protein of Plasmodium: a mechanism of immune evasion by the malaria parasite?Bull World Health Organ. 1990;68 Suppl(Suppl):66-73. Bull World Health Organ. 1990. PMID: 1709835 Free PMC article. Review.
Cited by
-
A human monoclonal antibody blocks malaria transmission and defines a highly conserved neutralizing epitope on gametes.Nat Commun. 2021 Mar 19;12(1):1750. doi: 10.1038/s41467-021-21955-1. Nat Commun. 2021. PMID: 33741942 Free PMC article.
-
Injectable anti-malarials revisited: discovery and development of new agents to protect against malaria.Malar J. 2018 Nov 1;17(1):402. doi: 10.1186/s12936-018-2549-1. Malar J. 2018. PMID: 30384848 Free PMC article. Review.
-
How to induce protective humoral immunity against Plasmodium falciparum circumsporozoite protein.J Exp Med. 2022 Feb 7;219(2):e20201313. doi: 10.1084/jem.20201313. Epub 2022 Jan 10. J Exp Med. 2022. PMID: 35006242 Free PMC article. Review.
-
Guiding the Immune Response to a Conserved Epitope in MSP2, an Intrinsically Disordered Malaria Vaccine Candidate.Vaccines (Basel). 2021 Aug 4;9(8):855. doi: 10.3390/vaccines9080855. Vaccines (Basel). 2021. PMID: 34451980 Free PMC article.
-
Human antibodies against noncircumsporozoite proteins block Plasmodium falciparum parasite development in hepatocytes.JCI Insight. 2022 Mar 22;7(6):e153524. doi: 10.1172/jci.insight.153524. JCI Insight. 2022. PMID: 35167490 Free PMC article.
References
-
- Aikawa M, Yoshida N, Nussenzweig RS, Nussenzweig V. The Protective Antigen of Malarial Sporozoites (Plasmodium berghei) Is a Differentiation Antigen. J Immunol. 1981;126:2494–2495. - PubMed
-
- Aricescu AR, Lu W, Jones EY. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr Sect D Biol Crystallogr. 2006;62:1243–1250. - PubMed
-
- Behet MC, Foquet L, van Gemert GJ, Bijker EM, Meuleman P, Leroux-Roels G, Hermsen CC, Scholzen A, Sauerwein RW. Sporozoite immunization of human volunteers under chemoprophylaxis induces functional antibodies against pre-erythrocytic stages of Plasmodium falciparum. Malar J. 2014;13:1–12. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

