Skin Immune Landscape: Inside and Outside the Organism
- PMID: 29180836
- PMCID: PMC5664322
- DOI: 10.1155/2017/5095293
Skin Immune Landscape: Inside and Outside the Organism
Abstract
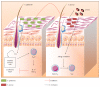
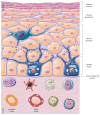
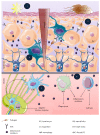
The skin is an essential organ to the human body protecting it from external aggressions and pathogens. Over the years, the skin was proven to have a crucial immunological role, not only being a passive protective barrier but a network of effector cells and molecular mediators that constitute a highly sophisticated compound known as the "skin immune system" (SIS). Studies of skin immune sentinels provided essential insights of a complex and dynamic immunity, which was achieved through interaction between the external and internal cutaneous compartments. In fact, the skin surface is cohabited by microorganisms recognized as skin microbiota that live in complete harmony with the immune sentinels and contribute to the epithelial barrier reinforcement. However, under stress, the symbiotic relationship changes into a dysbiotic one resulting in skin disorders. Hence, the skin microbiota may have either positive or negative influence on the immune system. This review aims at providing basic background information on the cutaneous immune system from major cellular and molecular players and the impact of its microbiota on the well-coordinated immune responses in host defense.
Figures

Similar articles
-
The Role of Cutaneous Microbiota Harmony in Maintaining a Functional Skin Barrier.J Drugs Dermatol. 2017 Jan 1;16(1):12-18. J Drugs Dermatol. 2017. PMID: 28095528
-
How the innate immune system trains immunity: lessons from studying atopic dermatitis and cutaneous bacteria.J Dtsch Dermatol Ges. 2016 Feb;14(2):153-6. doi: 10.1111/ddg.12843. Epub 2016 Jan 20. J Dtsch Dermatol Ges. 2016. PMID: 26788792 Review.
-
The role of innate immune signaling in the pathogenesis of atopic dermatitis and consequences for treatments.Semin Immunopathol. 2016 Jan;38(1):29-43. doi: 10.1007/s00281-015-0544-y. Epub 2015 Nov 16. Semin Immunopathol. 2016. PMID: 26573298 Review.
-
Novel mechanisms of microbial crosstalk with skin innate immunity.Exp Dermatol. 2021 Oct;30(10):1484-1495. doi: 10.1111/exd.14429. Epub 2021 Jul 21. Exp Dermatol. 2021. PMID: 34252227 Review.
-
The skin microbiome: potential for novel diagnostic and therapeutic approaches to cutaneous disease.Semin Cutan Med Surg. 2014 Jun;33(2):98-103. doi: 10.12788/j.sder.0087. Semin Cutan Med Surg. 2014. PMID: 25085669 Free PMC article. Review.
Cited by
-
Trauma of Peripheral Innervation Impairs Content of Epidermal Langerhans Cells.Diagnostics (Basel). 2022 Feb 23;12(3):567. doi: 10.3390/diagnostics12030567. Diagnostics (Basel). 2022. PMID: 35328120 Free PMC article.
-
A Pilot Study to Explore a Correlation between Inflammatory Markers and the Wound Healing Rate in Diabetic Patients.Medicina (Kaunas). 2022 Mar 6;58(3):390. doi: 10.3390/medicina58030390. Medicina (Kaunas). 2022. PMID: 35334566 Free PMC article.
-
Isolation of Skin Leukocytes Uncovers Phagocyte Inflammatory Responses During Induction and Resolution of Cutaneous Inflammation in Fish.Front Immunol. 2021 Sep 24;12:725063. doi: 10.3389/fimmu.2021.725063. eCollection 2021. Front Immunol. 2021. PMID: 34630399 Free PMC article.
-
Daily very low UV dose exposure enhances adaptive immunity, compared with a single high-dose exposure. Consequences for the control of a skin infection.Immunology. 2018 Jul;154(3):510-521. doi: 10.1111/imm.12901. Epub 2018 Feb 15. Immunology. 2018. PMID: 29377107 Free PMC article.
-
Skin Cancer Microenvironment: What We Can Learn from Skin Aging?Int J Mol Sci. 2023 Sep 13;24(18):14043. doi: 10.3390/ijms241814043. Int J Mol Sci. 2023. PMID: 37762344 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

