Virus replicon particle vaccines expressing nucleoprotein of influenza A virus mediate enhanced inflammatory responses in pigs
- PMID: 29180817
- PMCID: PMC5703990
- DOI: 10.1038/s41598-017-16419-w
Virus replicon particle vaccines expressing nucleoprotein of influenza A virus mediate enhanced inflammatory responses in pigs
Abstract
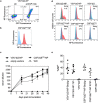
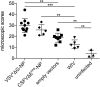
Studies in the mouse model indicate that the nucleoprotein of influenza A virus represents an interesting vaccine antigen being well conserved across subtypes of influenza virus but still able to induce protective immune responses. Here we show that immunizations of pigs with vesicular stomatitis virus- and classical swine fever virus-derived replicon (VRP) particles expressing the nucleoprotein (NP) of H1N1 A/swine/Belzig/2/01 induced potent antibody and T-cell responses against influenza A virus. In contrast to a conventional whole inactivated virus vaccine, the VRP vaccines induced both NP-specific CD4 and CD8 T cells responses, including interferon-γ and tumor-necrosis-factor dual-secreting cell. Although T-cells and antibody responses were cross-reactive with the heterologous H1N2 A/swine/Bakum/R757/2010 challenge virus, they did not provide protection against infection. Surprisingly, vaccinated pigs showed enhanced virus shedding, lung inflammation and increased levels of systemic and lung interferon-α as well as elevated lung interleukin-6. In conclusion, our study shows that NP, although efficacious in the mouse model, appears not to be a promising stand-alone vaccine antigen for pigs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Intranasal Delivery of Inactivated Influenza Virus and Poly(I:C) Adsorbed Corn-Based Nanoparticle Vaccine Elicited Robust Antigen-Specific Cell-Mediated Immune Responses in Maternal Antibody Positive Nursery Pigs.Front Immunol. 2020 Dec 16;11:596964. doi: 10.3389/fimmu.2020.596964. eCollection 2020. Front Immunol. 2020. PMID: 33391267 Free PMC article.
-
Polyanhydride nanovaccine against swine influenza virus in pigs.Vaccine. 2017 Feb 22;35(8):1124-1131. doi: 10.1016/j.vaccine.2017.01.019. Epub 2017 Jan 20. Vaccine. 2017. PMID: 28117173
-
Mucosal Immunity and Protective Efficacy of Intranasal Inactivated Influenza Vaccine Is Improved by Chitosan Nanoparticle Delivery in Pigs.Front Immunol. 2018 May 2;9:934. doi: 10.3389/fimmu.2018.00934. eCollection 2018. Front Immunol. 2018. PMID: 29770135 Free PMC article.
-
Failure of protection and enhanced pneumonia with a US H1N2 swine influenza virus in pigs vaccinated with an inactivated classical swine H1N1 vaccine.Vet Microbiol. 2008 Jan 25;126(4):310-23. doi: 10.1016/j.vetmic.2007.07.011. Epub 2007 Jul 17. Vet Microbiol. 2008. PMID: 17719188
-
Protection of weaned pigs by vaccination with human adenovirus 5 recombinant viruses expressing the hemagglutinin and the nucleoprotein of H3N2 swine influenza virus.Vaccine. 2004 Sep 3;22(25-26):3427-34. doi: 10.1016/j.vaccine.2004.02.040. Vaccine. 2004. PMID: 15308368
Cited by
-
T and B Cell Immune Responses to Influenza Viruses in Pigs.Front Immunol. 2019 Feb 5;10:98. doi: 10.3389/fimmu.2019.00098. eCollection 2019. Front Immunol. 2019. PMID: 30804933 Free PMC article. Review.
-
Reduced Virus Load in Lungs of Pigs Challenged with Porcine Reproductive and Respiratory Syndrome Virus after Vaccination with Virus Replicon Particles Encoding Conserved PRRSV Cytotoxic T-Cell Epitopes.Vaccines (Basel). 2021 Mar 2;9(3):208. doi: 10.3390/vaccines9030208. Vaccines (Basel). 2021. PMID: 33801369 Free PMC article.
-
Cross-Protective Potential and Protection-Relevant Immune Mechanisms of Whole Inactivated Influenza Virus Vaccines Are Determined by Adjuvants and Route of Immunization.Front Immunol. 2019 Mar 29;10:646. doi: 10.3389/fimmu.2019.00646. eCollection 2019. Front Immunol. 2019. PMID: 30984200 Free PMC article.
-
Influenza A Virus in Swine: Epidemiology, Challenges and Vaccination Strategies.Front Vet Sci. 2020 Sep 22;7:647. doi: 10.3389/fvets.2020.00647. eCollection 2020. Front Vet Sci. 2020. PMID: 33195504 Free PMC article. Review.
-
Self-Amplifying Pestivirus Replicon RNA Encoding Influenza Virus Nucleoprotein and Hemagglutinin Promote Humoral and Cellular Immune Responses in Pigs.Front Immunol. 2021 Jan 28;11:622385. doi: 10.3389/fimmu.2020.622385. eCollection 2020. Front Immunol. 2021. PMID: 33584723 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

