Effects of IFN-γ coding plasmid supplementation in the immune response and protection elicited by Trypanosoma cruzi attenuated parasites
- PMID: 29178839
- PMCID: PMC5702110
- DOI: 10.1186/s12879-017-2834-6
Effects of IFN-γ coding plasmid supplementation in the immune response and protection elicited by Trypanosoma cruzi attenuated parasites
Abstract
Background: Previous studies showed that a naturally attenuated strain from Trypanosoma cruzi triggers an immune response mainly related to a Th2-type profile. Albeit this, a strong protection against virulent challenge was obtained after priming mice with this attenuated strain. However, this protection is not enough to completely clear parasites from the host. In T. cruzi infection, early Interferon-gamma (IFN-γ) is critical to lead type 1 responses able to control intracellular parasites. Therefore we evaluated whether the co-administration of a plasmid encoding murine IFN-γ could modify the immune response induced by infection with attenuated parasites and improve protection against further infections.
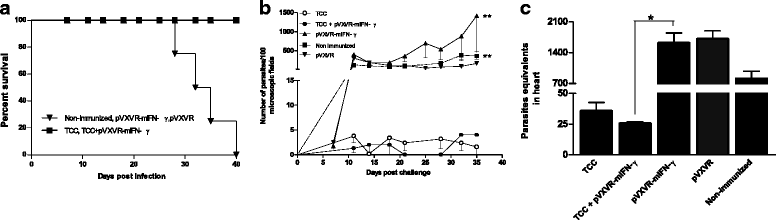
Methods: C57BL/6J mice were infected intraperitoneally with three doses of live attenuated parasites in combination with plasmid pVXVR-mIFN-γ. Before each infection dose, sera samples were collected for parasite specific antibodies determination and cytokine quantification. To evaluate the recall response to T. cruzi, mice were challenged with virulent parasites 30 days after the last dose and parasite load in peripheral blood and heart was evaluated.
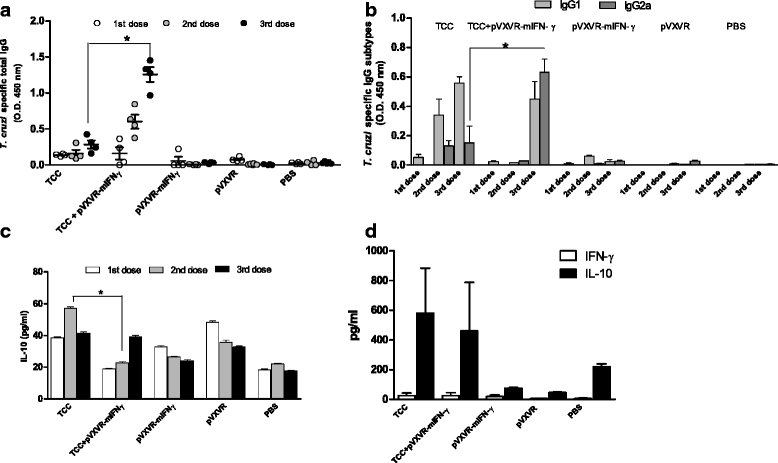
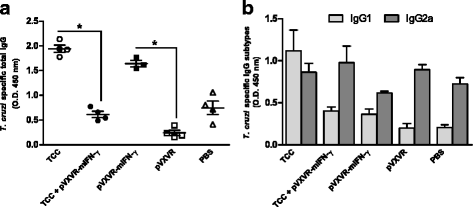
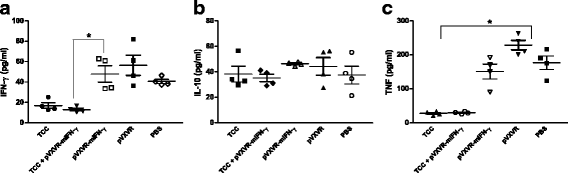
Results: As determined by ELISA, significantly increase in T. cruzi specific antibodies response was detected in the group in which pVXVR-mIFN-γ was incorporated, with a higher predominance of IgG2a subtype in comparison to the group of mice only inoculated with attenuated parasites. At our limit of detection, serum levels of IFN-γ were not detected, however a slight decrease in IL-10 concentrations was observed in groups in which pVXVR-mIFN-γ was supplemented. To analyze if the administration of pVXVR-mIFN-γ has any beneficial effect in protection against subsequent infections, all experimental groups were submitted to a lethal challenge with virulent bloodstream trypomastigotes. Similar levels of challenge parasites were detected in peripheral blood and heart of mice primed with attenuated parasites alone or combined with plasmid DNA. Expansion of IgG antibodies was not significant in TCC+ pVXVR-mIFN-γ; however, the overall tendency to sustain a Th2 profile was maintained.
Conclusions: Overall, these results suggest that administration of plasmid pVXVR-mIFN-γ could have beneficial effects on host specific antibody production in response to T. cruzi attenuated infection; however, this outcome is not reflected in an improved protection against further virulent infections.
Keywords: Attenuated infection; IFN-γ; Trypanosoma cruzi.
Conflict of interest statement
Ethics approval
All animal protocols adhered to the National Institutes of Health (NIH) “Guide for the care and use of laboratory animals” and were approved by the School of Health Sciences and by the Ethical Committee of the National University of Salta, Argentina (N° 014–2011).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Evaluation of pathogen P21 protein as a potential modulator of the protective immunity induced by Trypanosoma cruzi attenuated parasites.Mem Inst Oswaldo Cruz. 2019;114:e180571. doi: 10.1590/0074-02760180571. Epub 2019 May 20. Mem Inst Oswaldo Cruz. 2019. PMID: 31116244 Free PMC article.
-
Challenge of chronically infected mice with homologous trypanosoma cruzi parasites enhances the immune response but does not modify cardiopathy: implications for the design of a therapeutic vaccine.Clin Vaccine Immunol. 2013 Feb;20(2):248-54. doi: 10.1128/CVI.00032-12. Epub 2012 Dec 19. Clin Vaccine Immunol. 2013. PMID: 23254299 Free PMC article.
-
Levels of tumor necrosis factor alpha, gamma interferon, and interleukins 4,6, and 10 as determined in mice infected with virulent or attenuated strains of Trypanosoma cruzi.Parasitol Res. 1999 Feb;85(2):147-50. doi: 10.1007/s004360050524. Parasitol Res. 1999. PMID: 9934965
-
How are antibodies involved in the protective mechanism of susceptible mice infected with T. cruzi?Braz J Med Biol Res. 2000 Mar;33(3):253-8. doi: 10.1590/s0100-879x2000000300001. Braz J Med Biol Res. 2000. PMID: 10719375 Review.
-
Evasion of immune responses by Trypanosoma cruzi, the etiological agent of Chagas disease.Braz J Med Biol Res. 2011 Feb;44(2):84-90. doi: 10.1590/s0100-879x2011007500005. Epub 2011 Jan 14. Braz J Med Biol Res. 2011. PMID: 21243314 Review.
Cited by
-
The Case for the Development of a Chagas Disease Vaccine: Why? How? When?Trop Med Infect Dis. 2021 Jan 26;6(1):16. doi: 10.3390/tropicalmed6010016. Trop Med Infect Dis. 2021. PMID: 33530605 Free PMC article. Review.
-
Evaluation of pathogen P21 protein as a potential modulator of the protective immunity induced by Trypanosoma cruzi attenuated parasites.Mem Inst Oswaldo Cruz. 2019;114:e180571. doi: 10.1590/0074-02760180571. Epub 2019 May 20. Mem Inst Oswaldo Cruz. 2019. PMID: 31116244 Free PMC article.
-
Cruzipain and Its Physiological Inhibitor, Chagasin, as a DNA-Based Therapeutic Vaccine Against Trypanosoma cruzi.Front Immunol. 2020 Oct 9;11:565142. doi: 10.3389/fimmu.2020.565142. eCollection 2020. Front Immunol. 2020. PMID: 33162979 Free PMC article.
-
Protective Efficacy of the Epitope-Conjugated Antigen N-Tc52/TSkb20 in Mitigating Trypanosoma cruzi Infection through CD8+ T-Cells and IFNγ Responses.Vaccines (Basel). 2024 Jun 4;12(6):621. doi: 10.3390/vaccines12060621. Vaccines (Basel). 2024. PMID: 38932350 Free PMC article.
-
Exploring the performance of Escherichia coli outer membrane vesicles as a tool for vaccine development against Chagas disease.Mem Inst Oswaldo Cruz. 2023 May 22;118:e220263. doi: 10.1590/0074-02760220263. eCollection 2023. Mem Inst Oswaldo Cruz. 2023. PMID: 37222309 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

