Src family kinase tyrosine phosphorylates Toll-like receptor 4 to dissociate MyD88 and Mal/Tirap, suppressing LPS-induced inflammatory responses
- PMID: 29175418
- PMCID: PMC5733702
- DOI: 10.1016/j.bcp.2017.11.015
Src family kinase tyrosine phosphorylates Toll-like receptor 4 to dissociate MyD88 and Mal/Tirap, suppressing LPS-induced inflammatory responses
Abstract
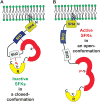
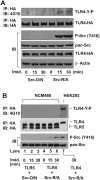
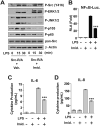
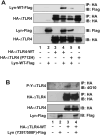
Src family kinases (SFKs) are a family of protein tyrosine kinases containing nine members: Src, Lyn, Fgr, Hck, Lck, Fyn, Blk, Yes, and Ylk. Although SFK activation is a major immediate signaling event in LPS/Toll-like receptor 4 (TLR4) signaling, its precise role has remained elusive due to various contradictory results obtained from a certain SFK member-deficient mice or cells. The observed inconsistencies may be due to the compensation or redundancy by other SFKs upon a SFK deficiency. The chemical rescuing approach was suggested to induce temporal and precise SFK activation in living cells, thereby limiting the chance of cellular adaption to a SFK-deficient condition. Using the rescuing approach, we demonstrate that restoring SFK activity not only induces tyrosine phosphorylation of TLR4, but also inhibits LPS-induced NFκB and JNK1/2 activation and consequently suppresses LPS-induced cytokine production. TLR4 normally recruits TIR domain-containing adaptors in response to LPS, however, temporally restored SFK activation disrupts the LPS-induced association of MyD88 and Mal/Tirap with TLR4. Additionally, using kinase-dead SFK-Lyn (Y397/508F) and constitutively active SFK-Lyn (Y508F), we found that the kinase-dead SFK inhibits TLR4 tyrosine phosphorylation with reduced binding affinity to TLR4, while the kinase-active SFK strongly binds to TLR4 and promotes TLR4 tyrosine phosphorylation, suggesting that SFK kinase activity is required for TLR4 tyrosine phosphorylation and TLR4-SFK interaction. Together, our results demonstrate that SFK activation induces TLR4 tyrosine phosphorylation, consequently dissociating MyD88 and Mal/Tirap from TLR4 and inhibiting LPS-induced inflammatory responses, suggesting a negative feedback loop regulated by SFK-induced tyrosine phosphorylation in TLR4.
Keywords: Chemical rescuing approach; Negative feedback; Sepsis; Src family kinase; Toll like receptor; Tyrosine phosphorylation.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
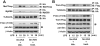


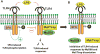
Similar articles
-
TRAF6 protein couples Toll-like receptor 4 signaling to Src family kinase activation and opening of paracellular pathway in human lung microvascular endothelia.J Biol Chem. 2012 May 11;287(20):16132-45. doi: 10.1074/jbc.M111.310102. Epub 2012 Mar 23. J Biol Chem. 2012. PMID: 22447928 Free PMC article.
-
Distinct pathways of LPS-induced NF-kappa B activation and cytokine production in human myeloid and nonmyeloid cells defined by selective utilization of MyD88 and Mal/TIRAP.Blood. 2004 Mar 15;103(6):2229-37. doi: 10.1182/blood-2003-04-1356. Epub 2003 Nov 20. Blood. 2004. PMID: 14630816
-
The regulation of the expression of inducible nitric oxide synthase by Src-family tyrosine kinases mediated through MyD88-independent signaling pathways of Toll-like receptor 4.Biochem Pharmacol. 2005 Oct 15;70(8):1231-40. doi: 10.1016/j.bcp.2005.07.020. Biochem Pharmacol. 2005. PMID: 16140274
-
Modulation of Toll-interleukin 1 receptor mediated signaling.J Mol Med (Berl). 2005 Apr;83(4):258-66. doi: 10.1007/s00109-004-0622-4. Epub 2005 Jan 21. J Mol Med (Berl). 2005. PMID: 15662540 Review.
-
Src family kinases: regulation of their activities, levels and identification of new pathways.Biochim Biophys Acta. 2008 Jan;1784(1):56-65. doi: 10.1016/j.bbapap.2007.08.012. Epub 2007 Aug 22. Biochim Biophys Acta. 2008. PMID: 17905674 Review.
Cited by
-
Autotaxin loss accelerates intestinal inflammation by suppressing TLR4-mediated immune responses.EMBO Rep. 2020 Oct 5;21(10):e49332. doi: 10.15252/embr.201949332. Epub 2020 Sep 1. EMBO Rep. 2020. PMID: 32875703 Free PMC article.
-
Dasatinib regulates LPS-induced microglial and astrocytic neuroinflammatory responses by inhibiting AKT/STAT3 signaling.J Neuroinflammation. 2019 Oct 26;16(1):190. doi: 10.1186/s12974-019-1561-x. J Neuroinflammation. 2019. PMID: 31655606 Free PMC article.
-
Screening of key genes related to the prognosis of mouse sepsis.Biosci Rep. 2020 Oct 30;40(10):BSR20202649. doi: 10.1042/BSR20202649. Biosci Rep. 2020. PMID: 33015708 Free PMC article.
-
Anti-Inflammatory Effects of Licania macrocarpa Cuatrec Methanol Extract Target Src- and TAK1-Mediated Pathways.Evid Based Complement Alternat Med. 2019 Sep 12;2019:4873870. doi: 10.1155/2019/4873870. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31611922 Free PMC article.
-
Protein-N-myristoylation-dependent phosphorylation of serine 13 of tyrosine kinase Lyn by casein kinase 1γ at the Golgi during intracellular protein traffic.Sci Rep. 2020 Oct 1;10(1):16273. doi: 10.1038/s41598-020-73248-0. Sci Rep. 2020. PMID: 33004926 Free PMC article.
References
-
- Hamerman JA, Lanier LL. Inhibition of immune responses by ITAM-bearing receptors. Sci STKE. 2006;2006:re1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

