Experience with etanercept, tocilizumab and interleukin-1 inhibitors in systemic onset juvenile idiopathic arthritis patients from the BIKER registry
- PMID: 29166924
- PMCID: PMC5700562
- DOI: 10.1186/s13075-017-1462-2
Experience with etanercept, tocilizumab and interleukin-1 inhibitors in systemic onset juvenile idiopathic arthritis patients from the BIKER registry
Abstract
Background: Treatment of systemic onset juvenile idiopathic arthritis JIA (sJIA), although dramatically improved, remains a challenge. Experience from clinical practice will be presented using data from the German Biologics register (BiKeR) for evaluation of efficacy and safety of treatment with etanercept (ETA), tocilizumab (TOC) and the interleukin-1 inhibitors anakinra and canakinumab (IL-1i) in sJIA.
Methods: Patients with sJIA documented in the BIKeR register, who were exposed to ETA, TOC or IL-1i were identified. Baseline demographics, clinical characteristics and disease activity parameters have been documented. Efficacy was determined using the JIA-American College of Rheumatology (ACR) response criteria and the Juvenile Disease Activity Score 10 (JADAS10). An intention-to-treat analysis was performed and patients who discontinued due to inefficacy or intolerance were analysed as non-responders. Safety assessments were based on adverse events (AEs) reports.
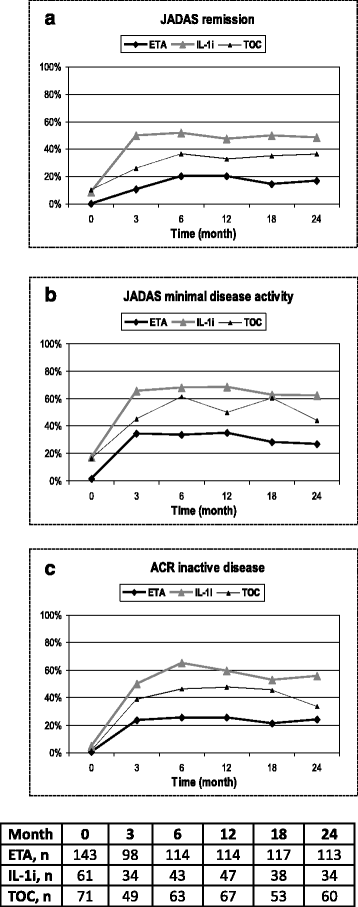
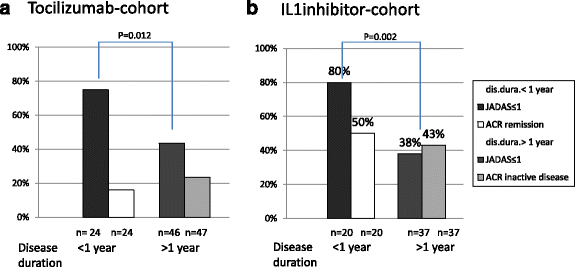
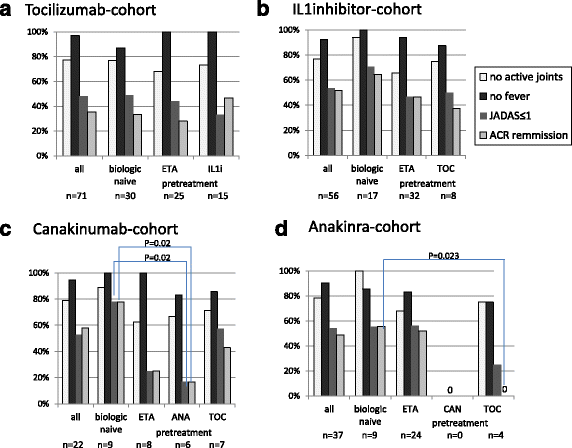
Results: Since 2000, 245 sJIA patients (50.3% male) exposed to biologic agents have been identified: 143 patients treated with ETA, 71 with TOC and 60 with IL-1i (anakinra 38, canakinumab 22). All patients received systemic steroids for pre-treatment but less frequently with TOC and IL-1i than with ETA for concomitant treatment. At baseline, the ETA cohort had fewer systemic disease manifestations but more active joints. The JIA-ACR 30/50/70/90 response over a period of 24 months was reached more often in the IL-1i and TOC cohort than with ETA. ETA/TOC/IL1i JADAS-remission (JADAS ≤1) was reached in 20%/37%/52%, minimal disease activity (JADAS ≤3.8 in 35%/61%/68% and ACR inactive disease in 24%/33%/56%). As compared to ETA, rates of AEs were significantly higher in the TOC cohort (risk ratio (RR) 5.3/patient-year; p < 0.0001) and serious AE were observed more frequently with TOC (RR 2.5; p < 0.5) and IL1i (2.9; p < 0.01).
Conclusions: A large proportion of patients gained significant response to treatment especially with TOC or IL-1is. After 6 months on treatment, JADAS remission was reached by up to half of patients while up to two thirds reached JADAS minimal disease activity. ETA has been used in the past but it is clearly less effective and its use in systemic JIA has markedly decreased in Germany.
Keywords: Anakinra; Canakinumab; Etanercept; Still’s disease; Systemic onset juvenile idiopathic arthritis; Tocilizumab.
Conflict of interest statement
Ethics approval and consent to participate
The study was conducted in accordance with the protocol, International Conference for Harmonization (ICH), Good Clinical Practice (GCP), FDA regulations governing clinical study conduct, ethical principles that have their origin in the Declaration of Helsinki, 1996 revision and 2000 revision with subsequent clarifications, and all applicable local regulations. Before the study was initiated, the study protocol, the informed consent form and subject information were submitted for review to the responsible independent ethics committee of the Aerztekammer Nordrhein, Duesseldorf, Germany, reference number 2/2015/7441. Parents/legal guardians signed the informed consent form before any study-related procedures occurred.
Consent for publication
Not applicable.
Competing interests
Gerd Horneff has received research funds, advisory board membership and honorary fees from Abbvie, Pfizer and Roche. Kirsten Minden has received research grants from Abbvie, Pfizer and Roche and honorary fees from Abbvie, Pfizer, Genzyme and Pharm-Allergan. Ivan Foeldvari has received personal fees from Abbvie, Chugai, Novartis and Genzyme. Anton Hospach has received advisory board membership and honoraria from Pfizer, Abbvie and Roche. Schulz AC, Klotsche J, Trauzeddel R, Ganser G., Weller-Heinemann F and Haas JP declare that they have no competing interests.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Long-term surveillance of biologic therapies in systemic-onset juvenile idiopathic arthritis: data from the German BIKER registry.Rheumatology (Oxford). 2020 Sep 1;59(9):2287-2298. doi: 10.1093/rheumatology/kez577. Rheumatology (Oxford). 2020. PMID: 31846042
-
Efficacy and safety of adalimumab as the first and second biologic agent in juvenile idiopathic arthritis: the German Biologics JIA Registry.Arthritis Rheumatol. 2014 Sep;66(9):2580-9. doi: 10.1002/art.38741. Arthritis Rheumatol. 2014. PMID: 24942886
-
Long-term safety and effectiveness of etanercept in JIA: an 18-year experience from the BiKeR registry.Arthritis Res Ther. 2020 Oct 29;22(1):258. doi: 10.1186/s13075-020-02326-5. Arthritis Res Ther. 2020. PMID: 33121528 Free PMC article.
-
Efficacy and safety of biological agents for systemic juvenile idiopathic arthritis: a systematic review and meta-analysis of randomized trials.Rheumatology (Oxford). 2016 Apr;55(4):669-79. doi: 10.1093/rheumatology/kev382. Epub 2015 Nov 30. Rheumatology (Oxford). 2016. PMID: 26628580 Free PMC article. Review.
-
The clinical effectiveness and cost-effectiveness of abatacept, adalimumab, etanercept and tocilizumab for treating juvenile idiopathic arthritis: a systematic review and economic evaluation.Health Technol Assess. 2016 Apr;20(34):1-222. doi: 10.3310/hta20340. Health Technol Assess. 2016. PMID: 27135404 Free PMC article. Review.
Cited by
-
Inhibition of uric acid or IL-1β ameliorates respiratory syncytial virus immunopathology and development of asthma.Allergy. 2020 Sep;75(9):2279-2293. doi: 10.1111/all.14310. Epub 2020 May 15. Allergy. 2020. PMID: 32277487 Free PMC article.
-
Efficacy and Safety of Etanercept Biosimilars Compared With the Originator for Treatment of Juvenile Arthritis: A Prospective Observational Study.ACR Open Rheumatol. 2021 Nov;3(11):779-787. doi: 10.1002/acr2.11325. Epub 2021 Aug 27. ACR Open Rheumatol. 2021. PMID: 34449981 Free PMC article.
-
Juvenile idiopathic arthritis with systemic onset with inflammatory bone lesions: two case reports of patients successfully treated with canakinumab and experience gained from literature.Front Pediatr. 2023 May 31;11:1163483. doi: 10.3389/fped.2023.1163483. eCollection 2023. Front Pediatr. 2023. PMID: 37325364 Free PMC article.
-
The Therapy of SARS-CoV-2 Infection in Children.J Clin Med. 2023 Dec 25;13(1):120. doi: 10.3390/jcm13010120. J Clin Med. 2023. PMID: 38202127 Free PMC article. Review.
-
American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 3.Arthritis Rheumatol. 2022 Apr;74(4):e1-e20. doi: 10.1002/art.42062. Epub 2022 Feb 3. Arthritis Rheumatol. 2022. PMID: 35118829 Free PMC article.
References
-
- Janow G, Schanberg LE, Setoguchi S, Hasselblad V, Mellins ED, Schneider R, Kimura Y, the CARRA Legacy Registry Investigators The Systemic Juvenile Idiopathic Arthritis Cohort of the Childhood Arthritis and Rheumatology Research Alliance Registry: 2010–2013. J Rheumatol. 2016;43:1755–62. doi: 10.3899/jrheum.150997. - DOI - PubMed
-
- Ilowite NT, Laxer RM. Pharmacology and drug therapy. In: Cassidy JT, Petty RE, Laxer RM, Lindsley CB, editors. Textbook of pediatric rheumatology. 6. Philadelphia: Saunders Elsevier; 2011. pp. 71–126.
-
- Kimura Y, Pinho P, Walco G, Higgins G, Hummell D, Szer I, et al. Etanercept treatment in patients with refractory systemic onset juvenile rheumatoid arthritis. J Rheumatol. 2005;32:935–42. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

