Exposure to Concentrated Ambient PM2.5 Compromises Spermatogenesis in a Mouse Model: Role of Suppression of Hypothalamus-Pituitary-Gonads Axis
- PMID: 29165613
- PMCID: PMC6059119
- DOI: 10.1093/toxsci/kfx261
Exposure to Concentrated Ambient PM2.5 Compromises Spermatogenesis in a Mouse Model: Role of Suppression of Hypothalamus-Pituitary-Gonads Axis
Abstract
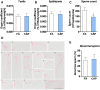
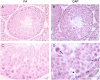
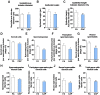
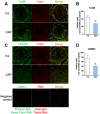
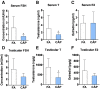
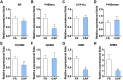
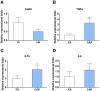
Epidemiological studies link ambient fine particulate matter (PM2.5) pollution to abnormalities in the male reproductive system. However, few toxicological studies have investigated this potentially important adverse effect of PM2.5 pollution. Therefore, in the present study, we analyzed the effects of PM2.5 exposure on spermatogenesis and hypothalamic-pituitary-gonadal (HPG) axis in a murine model. Fourteen male C57BL/6J mice were subjected to a 4-month exposure to filtered air or concentrated ambient PM2.5 (CAP). Their sperm count, testicular histology, spermatogenic parameters, and the major components of HPG axis were assessed. Exposure to CAP significantly reduced sperm count in the epididymis. This was accompanied by Sertoli cell vacuolization, immature germ cell dislocation, and decreases in pachytene spermatocytes and round spermatids of stage VII seminiferous tubules, suggesting a marked impairment of spermatogenesis in these mice. This impairment of spermatogenesis appeared to be attributable to a suppression of HPG axis subsequent to CAP exposure-induced hypothalamic inflammation, as exposure to CAP significantly increased TNFα and IL1b mRNA levels and meanwhile decreased gonadotropin-releasing hormone mRNA expression in the hypothalamus. Moreover, CAP exposure significantly reduced circulating testosterone and follicle-stimulating hormone, testicular testosterone and mRNA expression of follicle-stimulating hormone target gene SHBG and luteinizing hormone target genes P450scc, 17βHSD, and StAR. The present data demonstrate that exposure to ambient PM2.5 impairs spermatogenesis in murine model, raising the concern over effects of ambient PM2.5 pollution on the male reproductive function.
Figures
Similar articles
-
Hypothalamic-pituitary-adrenal axis mediates ambient PM2.5 exposure-induced pulmonary inflammation.Ecotoxicol Environ Saf. 2021 Jan 15;208:111464. doi: 10.1016/j.ecoenv.2020.111464. Epub 2020 Oct 16. Ecotoxicol Environ Saf. 2021. PMID: 33075589 Free PMC article.
-
Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic-pituitary-testicular axis.Toxicology. 2015 Mar 2;329:1-9. doi: 10.1016/j.tox.2015.01.002. Epub 2015 Jan 6. Toxicology. 2015. PMID: 25575453
-
Exposure of Mice during Spermatogenesis: A Role of Inhibitor Kinase 2 in Pro-Opiomelanocortin Neurons.Environ Health Perspect. 2021 Sep;129(9):97006. doi: 10.1289/EHP8868. Epub 2021 Sep 8. Environ Health Perspect. 2021. PMID: 34495743 Free PMC article.
-
The adverse effects of psychotropic drugs as an endocrine disrupting chemicals on the hypothalamic-pituitary regulation in male.Life Sci. 2020 Jul 15;253:117704. doi: 10.1016/j.lfs.2020.117704. Epub 2020 Apr 24. Life Sci. 2020. PMID: 32339542 Review.
-
Endocrinology of male infertility.Br Med Bull. 1979 May;35(2):187-92. doi: 10.1093/oxfordjournals.bmb.a071568. Br Med Bull. 1979. PMID: 387166 Review.
Cited by
-
Intersection of Aging and Particulate Matter 2.5 Exposure in Real World: Effects on Inflammation and Endocrine Axis Activities in Rats.Int J Endocrinol. 2024 Jun 27;2024:8501696. doi: 10.1155/2024/8501696. eCollection 2024. Int J Endocrinol. 2024. PMID: 38966821 Free PMC article.
-
Indirect mediators of systemic health outcomes following nanoparticle inhalation exposure.Pharmacol Ther. 2022 Jul;235:108120. doi: 10.1016/j.pharmthera.2022.108120. Epub 2022 Jan 24. Pharmacol Ther. 2022. PMID: 35085604 Free PMC article. Review.
-
Aspirin Alleviates Particulate Matter Induced Asymptomatic Orchitis of Mice via Suppression of cGAS-STING Signaling.Front Immunol. 2021 Dec 1;12:734546. doi: 10.3389/fimmu.2021.734546. eCollection 2021. Front Immunol. 2021. PMID: 34925318 Free PMC article.
-
Identification and characterization of circular RNA in the model of autism spectrum disorder from PM2.5 exposure.Front Genet. 2023 May 9;14:970465. doi: 10.3389/fgene.2023.970465. eCollection 2023. Front Genet. 2023. PMID: 37229188 Free PMC article.
-
Environmental Factors-Induced Oxidative Stress: Hormonal and Molecular Pathway Disruptions in Hypogonadism and Erectile Dysfunction.Antioxidants (Basel). 2021 May 24;10(6):837. doi: 10.3390/antiox10060837. Antioxidants (Basel). 2021. PMID: 34073826 Free PMC article. Review.
References
-
- Agency U. S. E. P. (2013). National ambient air quality standards for particulate matter. Final rule. Fed. Regist. 78, 3086–3287.
-
- Ahmed E. A., de Rooij D. G. (2009). Staging of mouse seminiferous tubule cross-sections. Methods Mol. Biol. 558, 263–277. - PubMed
-
- Cao X. N., Yan C., Liu D. Y., Peng J. P., Chen J. J., Zhou Y., Long C. L., He D. W., Lin T., Shen L. J. (2015). Fine particulate matter leads to reproductive impairment in male rats by overexpressing phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway. Toxicol. Lett. 237, 181–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

