Development of Peritoneal Carcinomatosis in Epithelial Ovarian Cancer: A Review
- PMID: 29164988
- PMCID: PMC5794203
- DOI: 10.1369/0022155417742897
Development of Peritoneal Carcinomatosis in Epithelial Ovarian Cancer: A Review
Abstract
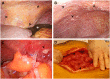
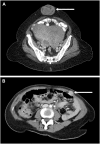
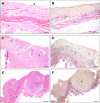
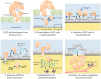
Epithelial ovarian cancer (EOC) metastasizes intra-abdominally with often numerous, superficial, small-sized lesions. This so-called peritoneal carcinomatosis is difficult to treat, and peritoneal recurrences are frequently observed, leading to a poor prognosis. Underlying mechanisms of interactions between EOC and peritoneal cells are incompletely understood. This review summarizes and discusses the development of peritoneal carcinomatosis from a cell-biological perspective, focusing on characteristics of EOC and peritoneal cells. We aim to provide insight into how peritoneum facilitates tumor adhesion but limits size of lesions and depth of invasion. The development of peritoneal carcinomatosis is a multistep process that requires adaptations of EOC and peritoneal cells. Mechanisms that enable tumor adhesion and growth involve cadherin restructuring on EOC cells, integrin-mediated adhesion, and mesothelial evasion by mechanical forces driven by integrin-ligand interactions. Clinical trials targeting these mechanisms, however, showed only limited effects. Other factors that inhibit tumor growth and deep invasion are virtually unknown. Future studies are needed to elucidate the exact mechanisms that underlie the development and limited growth of peritoneal carcinomatosis. This review on development of peritoneal carcinomatosis of EOC summarizes the current knowledge and its limitations. Clarification of the stepwise process may inspire future research to investigate new treatment approaches of peritoneal carcinomatosis.
Keywords: epithelial ovarian carcinoma; pathogenesis; peritoneal barrier; peritoneal metastases; peritoneal metastasis; peritoneum.
Conflict of interest statement
Figures


Similar articles
-
Poor perfusion of the microvasculature in peritoneal metastases of ovarian cancer.Clin Exp Metastasis. 2020 Apr;37(2):293-304. doi: 10.1007/s10585-020-10024-4. Epub 2020 Feb 1. Clin Exp Metastasis. 2020. PMID: 32008138 Free PMC article.
-
HDAC1/2 control mesothelium/ovarian cancer adhesive interactions impacting on Talin-1-α5β1-integrin-mediated actin cytoskeleton and extracellular matrix protein remodeling.J Exp Clin Cancer Res. 2024 Jan 23;43(1):27. doi: 10.1186/s13046-023-02930-8. J Exp Clin Cancer Res. 2024. PMID: 38254102 Free PMC article.
-
CCL2 secreted from cancer-associated mesothelial cells promotes peritoneal metastasis of ovarian cancer cells through the P38-MAPK pathway.Clin Exp Metastasis. 2020 Feb;37(1):145-158. doi: 10.1007/s10585-019-09993-y. Epub 2019 Sep 21. Clin Exp Metastasis. 2020. PMID: 31541326
-
[Intraperitoneal invasiveness of ovarian cancer from the cellular and molecular perspective].Ginekol Pol. 2015 Oct;86(10):782-6. doi: 10.17772/gp/58751. Ginekol Pol. 2015. PMID: 26677589 Review. Polish.
-
[Peritoneal carcinomatosis].Acta Med Port. 1998 Jun;11(6):585-9. Acta Med Port. 1998. PMID: 9773541 Review. Portuguese.
Cited by
-
Mid-Term Audit of a National Peritoneal Surface Malignancy Program Implementation in a Low Middle Income Country: The Moroccan Experience.Cancers (Basel). 2021 Mar 3;13(5):1088. doi: 10.3390/cancers13051088. Cancers (Basel). 2021. PMID: 33802609 Free PMC article.
-
Histological regression of peritoneal metastases of recurrent tubo-ovarian cancer after systemic chemotherapy.Front Surg. 2022 Sep 23;9:936613. doi: 10.3389/fsurg.2022.936613. eCollection 2022. Front Surg. 2022. PMID: 36338656 Free PMC article.
-
Intraperitoneal Chemotherapy for Peritoneal Metastases: Technical Innovations, Preclinical and Clinical Advances and Future Perspectives.Biology (Basel). 2021 Mar 15;10(3):225. doi: 10.3390/biology10030225. Biology (Basel). 2021. PMID: 33804167 Free PMC article. Review.
-
Polyunsaturated fatty acids promote M2-like TAM deposition via dampening RhoA-YAP1 signaling in the ovarian cancer microenvironment.Exp Hematol Oncol. 2024 Aug 28;13(1):90. doi: 10.1186/s40164-024-00558-8. Exp Hematol Oncol. 2024. PMID: 39198883 Free PMC article.
-
Investigating the mechanisms of peritoneal metastasis in gastric adenocarcinoma using a novel ex vivo peritoneal explant model.Sci Rep. 2022 Jul 7;12(1):11499. doi: 10.1038/s41598-022-13948-x. Sci Rep. 2022. PMID: 35798764 Free PMC article.
References
-
- Gerestein CG, Eijkemans MJ, de Jong D, van der Burg ME, Dykgraaf RH, Kooi GS, Baalbergen A, Burger CW, Ansink AC. The prediction of progression-free and overall survival in women with an advanced stage of epithelial ovarian carcinoma. BJOG. 2009;116(3):372–80. doi:10.1111/j.1471-0528.2008.02033.x. - DOI - PubMed
-
- Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R; Gynecologic Oncology Group. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–200. doi:10.1200/JCO.2003.02.153. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

