The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis
- PMID: 29161262
- PMCID: PMC5697827
- DOI: 10.1371/journal.pmed.1002446
The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis
Abstract
Background: The specificity of nucleic acid amplification tests (NAATs) used for early infant diagnosis (EID) of HIV infection is <100%, leading some HIV-uninfected infants to be incorrectly identified as HIV-infected. The World Health Organization recommends that infants undergo a second NAAT to confirm any positive test result, but implementation is limited. Our objective was to determine the impact and cost-effectiveness of confirmatory HIV testing for EID programmes in South Africa.
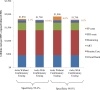
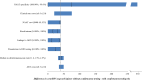
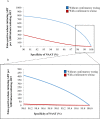
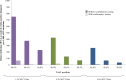
Method and findings: Using the Cost-effectiveness of Preventing AIDS Complications (CEPAC)-Pediatric model, we simulated EID testing at age 6 weeks for HIV-exposed infants without and with confirmatory testing. We assumed a NAAT cost of US$25, NAAT specificity of 99.6%, NAAT sensitivity of 100% for infants infected in pregnancy or at least 4 weeks prior to testing, and a mother-to-child transmission (MTCT) rate at 12 months of 4.9%; we simulated guideline-concordant rates of testing uptake, result return, and antiretroviral therapy (ART) initiation (100%). After diagnosis, infants were linked to and retained in care for 10 years (false-positive) or lifelong (true-positive). All parameters were varied widely in sensitivity analyses. Outcomes included number of infants with false-positive diagnoses linked to ART per 1,000 ART initiations, life expectancy (LE, in years) and per-person lifetime HIV-related healthcare costs. Both without and with confirmatory testing, LE was 26.2 years for HIV-infected infants and 61.4 years for all HIV-exposed infants; clinical outcomes for truly infected infants did not differ by strategy. Without confirmatory testing, 128/1,000 ART initiations were false-positive diagnoses; with confirmatory testing, 1/1,000 ART initiations were false-positive diagnoses. Because confirmatory testing averted costly HIV care and ART in truly HIV-uninfected infants, it was cost-saving: total cost US$1,790/infant tested, compared to US$1,830/infant tested without confirmatory testing. Confirmatory testing remained cost-saving unless NAAT cost exceeded US$400 or the HIV-uninfected status of infants incorrectly identified as infected was ascertained and ART stopped within 3 months of starting. Limitations include uncertainty in the data used in the model, which we examined with sensitivity and uncertainty analyses. We also excluded clinical harms to HIV-uninfected infants incorrectly treated with ART after false-positive diagnosis (e.g., medication toxicities); including these outcomes would further increase the value of confirmatory testing.
Conclusions: Without confirmatory testing, in settings with MTCT rates similar to that of South Africa, more than 10% of infants who initiate ART may reflect false-positive diagnoses. Confirmatory testing prevents inappropriate HIV diagnosis, is cost-saving, and should be adopted in all EID programmes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Optimizing infant HIV diagnosis with additional screening at immunization clinics in three sub-Saharan African settings: a cost-effectiveness analysis.J Int AIDS Soc. 2021 Jan;24(1):e25651. doi: 10.1002/jia2.25651. J Int AIDS Soc. 2021. PMID: 33474817 Free PMC article.
-
Point-of-care CD4 testing to inform selection of antiretroviral medications in south african antenatal clinics: a cost-effectiveness analysis.PLoS One. 2015 Mar 10;10(3):e0117751. doi: 10.1371/journal.pone.0117751. eCollection 2015. PLoS One. 2015. PMID: 25756498 Free PMC article.
-
Clinical Impact and Cost-effectiveness of Diagnosing HIV Infection During Early Infancy in South Africa: Test Timing and Frequency.J Infect Dis. 2016 Nov 1;214(9):1319-1328. doi: 10.1093/infdis/jiw379. Epub 2016 Aug 17. J Infect Dis. 2016. PMID: 27540110 Free PMC article.
-
Antiretroviral therapy (ART) for treating HIV infection in ART-eligible pregnant women.Cochrane Database Syst Rev. 2010 Mar 17;(3):CD008440. doi: 10.1002/14651858.CD008440. Cochrane Database Syst Rev. 2010. PMID: 20238370 Review.
-
Effectiveness of antiretroviral therapy in HIV-infected children under 2 years of age.Cochrane Database Syst Rev. 2012 Jul 11;(7):CD004772. doi: 10.1002/14651858.CD004772.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 May 22;(5):CD004772. doi: 10.1002/14651858.CD004772.pub4. PMID: 22786492 Updated. Review.
Cited by
-
Model-Based Methods to Translate Adolescent Medicine Trials Network for HIV/AIDS Interventions Findings Into Policy Recommendations: Rationale and Protocol for a Modeling Core (ATN 161).JMIR Res Protoc. 2019 Apr 16;8(4):e9898. doi: 10.2196/resprot.9898. JMIR Res Protoc. 2019. PMID: 30990464 Free PMC article.
-
The NSEBA Demonstration Project: implementation of a point-of-care platform for early infant diagnosis of HIV in rural Zambia.Trop Med Int Health. 2021 Sep;26(9):1036-1046. doi: 10.1111/tmi.13627. Epub 2021 Jun 7. Trop Med Int Health. 2021. PMID: 33999480 Free PMC article.
-
Risks and Benefits of Dolutegravir- and Efavirenz-Based Strategies for South African Women With HIV of Child-Bearing Potential: A Modeling Study.Ann Intern Med. 2019 May 7;170(9):614-625. doi: 10.7326/M18-3358. Epub 2019 Apr 2. Ann Intern Med. 2019. PMID: 30934067 Free PMC article.
-
Costs and cost-effectiveness of HIV early infant diagnosis in low- and middle-income countries: a scoping review.Infect Dis Poverty. 2022 Jul 15;11(1):82. doi: 10.1186/s40249-022-01006-7. Infect Dis Poverty. 2022. PMID: 35841117 Free PMC article.
-
Incorporating the HIV Infant Tracking System into standard-of-care early infant diagnosis of HIV services in Kenya: a cost-effectiveness analysis of the HITSystem randomised trial.Lancet Glob Health. 2023 Aug;11(8):e1217-e1224. doi: 10.1016/S2214-109X(23)00216-4. Lancet Glob Health. 2023. PMID: 37474229 Free PMC article. Clinical Trial.
References
-
- World Health Organization. 2015 progress report on the global plan towards the elimination of new HIV infections among children and keeping their mothers alive. Geneva: World Health Organization; 2015. [cited 2017 Oct 20]. Available from: http://www.unaids.org/sites/default/files/media_asset/JC2774_2015Progres....
-
- Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364(9441):1236–43. doi: 10.1016/S0140-6736(04)17140-7 - DOI - PubMed
-
- Ghadrshenas A, Ben Amor Y, Chang J, Dale H, Sherman G, Vojnov L. Improved access to early infant diagnosis is a critical part of a child-centric prevention of mother-to-child transmission agenda. AIDS. 2013;27(Supp 2):S197–205. - PubMed
-
- Mayaux M-J, Burgard M, Teglas J, Cottalorda J, Krivine A, Puel J, et al. Neonatal characteristics in rapidly progressive perinatally acquired HIV-1 disease. JAMA. 1996;275(8):606–10. - PubMed
-
- Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–44. doi: 10.1056/NEJMoa0800971 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

