Extracellular Vesicles in Cardiovascular Theranostics
- PMID: 29158817
- PMCID: PMC5695004
- DOI: 10.7150/thno.21274
Extracellular Vesicles in Cardiovascular Theranostics
Abstract
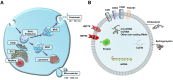
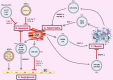
Extracellular vesicles (EVs) are small bilayer lipid membrane vesicles that can be released by most cell types and detected in most body fluids. EVs exert key functions for intercellular communication via transferring their bioactive cargos to recipient cells or activating signaling pathways in target cells. Increasing evidence has shown the important regulatory effects of EVs in cardiovascular diseases (CVDs). EVs secreted by cardiomyocytes, endothelial cells, fibroblasts, and stem cells play essential roles in pathophysiological processes such as cardiac hypertrophy, cardiomyocyte survival and apoptosis, cardiac fibrosis, and angiogenesis in relation to CVDs. In this review, we will first outline the current knowledge about the physical characteristics, biological contents, and isolation methods of EVs. We will then focus on the functional roles of cardiovascular EVs and their pathophysiological effects in CVDs, as well as summarize the potential of EVs as therapeutic agents and biomarkers for CVDs. Finally, we will discuss the specific application of EVs as a novel drug delivery system and the utility of EVs in the field of regenerative medicine.
Keywords: Extracellular vesicles; biomarkers.; cardiovascular diseases; exosomes; microvesicles; therapeutic agents.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Stem Cells-Derived Extracellular Vesicles: Potential Therapeutics for Wound Healing in Chronic Inflammatory Skin Diseases.Int J Mol Sci. 2021 Mar 19;22(6):3130. doi: 10.3390/ijms22063130. Int J Mol Sci. 2021. PMID: 33808520 Free PMC article. Review.
-
Circulating Extracellular Vesicles As Biomarkers and Drug Delivery Vehicles in Cardiovascular Diseases.Biomolecules. 2021 Mar 5;11(3):388. doi: 10.3390/biom11030388. Biomolecules. 2021. PMID: 33808038 Free PMC article. Review.
-
Cardioprotective role of extracellular vesicles: A highlight on exosome beneficial effects in cardiovascular diseases.J Cell Physiol. 2019 Dec;234(12):21732-21745. doi: 10.1002/jcp.28894. Epub 2019 May 29. J Cell Physiol. 2019. PMID: 31140622 Review.
-
Extracellular vesicles characteristics and emerging roles in atherosclerotic cardiovascular disease.Metabolism. 2018 Aug;85:213-222. doi: 10.1016/j.metabol.2018.04.008. Epub 2018 May 1. Metabolism. 2018. PMID: 29727628 Review.
-
Biology and Role of Extracellular Vesicles (EVs) in the Pathogenesis of Thrombosis.Int J Mol Sci. 2019 Jun 11;20(11):2840. doi: 10.3390/ijms20112840. Int J Mol Sci. 2019. PMID: 31212641 Free PMC article. Review.
Cited by
-
Cell-derived biomimetic nanoparticles as a novel drug delivery system for atherosclerosis: predecessors and perspectives.Regen Biomater. 2020 Aug;7(4):349-358. doi: 10.1093/rb/rbaa019. Epub 2020 May 25. Regen Biomater. 2020. PMID: 32793380 Free PMC article. Review.
-
Systemic proteomics and miRNA profile analysis of exosomes derived from human pluripotent stem cells.Stem Cell Res Ther. 2022 Sep 5;13(1):449. doi: 10.1186/s13287-022-03142-1. Stem Cell Res Ther. 2022. PMID: 36064647 Free PMC article.
-
Protective functions of Lycium barbarum polysaccharides in H2O2-injured vascular endothelial cells through anti-oxidation and anti-apoptosis effects.Biomed Rep. 2019 Nov;11(5):207-214. doi: 10.3892/br.2019.1240. Epub 2019 Sep 10. Biomed Rep. 2019. PMID: 31632668 Free PMC article.
-
Extracellular vesicle-based Nanotherapeutics: Emerging frontiers in anti-inflammatory therapy.Theranostics. 2020 Jul 9;10(18):8111-8129. doi: 10.7150/thno.47865. eCollection 2020. Theranostics. 2020. PMID: 32724461 Free PMC article. Review.
-
Extracellular vesicles in liver disease and beyond.World J Gastroenterol. 2018 Oct 28;24(40):4519-4526. doi: 10.3748/wjg.v24.i40.4519. World J Gastroenterol. 2018. PMID: 30386101 Free PMC article.
References
-
- Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166:189–97. - PubMed
-
- Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–88. - PubMed
-
- Dvorak HF, Quay SC, Orenstein NS, Dvorak AM, Hahn P, Bitzer AM. et al. Tumor shedding and coagulation. Science. 1981;212:923–4. - PubMed
-
- Taylor DD, Homesley HD, Doellgast GJ. Binding of specific peroxidase-labeled antibody to placental-type phosphatase on tumor-derived membrane fragments. Cancer Res. 1980;40:4064–9. - PubMed
-
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

