Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity
- PMID: 29158380
- PMCID: PMC5724266
- DOI: 10.1073/pnas.1710877114
Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity
Abstract
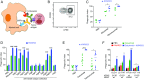
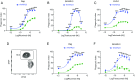
Cancer immunotherapy has emerged as a promising therapeutic intervention. However, complete and durable responses are only seen in a fraction of patients who have cancer. A key factor that limits therapeutic success is the infiltration of tumors by cells of the myeloid lineage. The inhibitory receptor signal regulatory protein-α (SIRPα) is a myeloid-specific immune checkpoint that engages the "don't eat me" signal CD47 expressed on tumors and normal tissues. We therefore developed the monoclonal antibody KWAR23, which binds human SIRPα with high affinity and disrupts its binding to CD47. Administered by itself, KWAR23 is inert, but given in combination with tumor-opsonizing monoclonal antibodies, KWAR23 greatly augments myeloid cell-dependent killing of a collection of hematopoietic and nonhematopoietic human tumor-derived cell lines. Following KWAR23 antibody treatment in a human SIRPA knockin mouse model, both neutrophils and macrophages infiltrate a human Burkitt's lymphoma xenograft and inhibit tumor growth, generating complete responses in the majority of treated animals. We further demonstrate that a bispecific anti-CD70/SIRPα antibody outperforms individually delivered antibodies in specific types of cancers. These studies demonstrate that SIRPα blockade induces potent antitumor activity by targeting multiple myeloid cell subsets that frequently infiltrate tumors. Thus, KWAR23 represents a promising candidate for combination therapy.
Keywords: SIRPA; bispecific antibody; cancer immunotherapy; humanized mouse; myeloid cells.
Conflict of interest statement
Conflict of interest statement: K.W., A.M.R., and I.L.W. are shareholders of Forty Seven, Inc., and have filed a patent application that describes the human anti-SIRPα antibody KWAR23. I.L.W. is co-inventor of the patent, and co-founder and director of the company that has licensed the antibody.
Figures


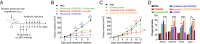
Similar articles
-
Anti-human SIRPα antibody is a new tool for cancer immunotherapy.Cancer Sci. 2018 May;109(5):1300-1308. doi: 10.1111/cas.13548. Epub 2018 Apr 15. Cancer Sci. 2018. PMID: 29473266 Free PMC article.
-
Cancer immunotherapy targeting the CD47/SIRPα axis.Eur J Cancer. 2017 May;76:100-109. doi: 10.1016/j.ejca.2017.02.013. Epub 2017 Mar 10. Eur J Cancer. 2017. PMID: 28286286 Review.
-
Targeting the myeloid checkpoint receptor SIRPα potentiates innate and adaptive immune responses to promote anti-tumor activity.J Hematol Oncol. 2020 Nov 30;13(1):160. doi: 10.1186/s13045-020-00989-w. J Hematol Oncol. 2020. PMID: 33256806 Free PMC article.
-
IgA-Mediated Killing of Tumor Cells by Neutrophils Is Enhanced by CD47-SIRPα Checkpoint Inhibition.Cancer Immunol Res. 2020 Jan;8(1):120-130. doi: 10.1158/2326-6066.CIR-19-0144. Epub 2019 Nov 5. Cancer Immunol Res. 2020. PMID: 31690649
-
The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer.Immunol Rev. 2017 Mar;276(1):145-164. doi: 10.1111/imr.12527. Immunol Rev. 2017. PMID: 28258703 Review.
Cited by
-
CD47-SIRPα blocking-based immunotherapy: Current and prospective therapeutic strategies.Clin Transl Med. 2022 Aug;12(8):e943. doi: 10.1002/ctm2.943. Clin Transl Med. 2022. PMID: 35908284 Free PMC article. Review.
-
Targeting CD47 in Sézary syndrome with SIRPαFc.Blood Adv. 2019 Apr 9;3(7):1145-1153. doi: 10.1182/bloodadvances.2018030577. Blood Adv. 2019. PMID: 30962222 Free PMC article. Clinical Trial.
-
Anti-human SIRPα antibody is a new tool for cancer immunotherapy.Cancer Sci. 2018 May;109(5):1300-1308. doi: 10.1111/cas.13548. Epub 2018 Apr 15. Cancer Sci. 2018. PMID: 29473266 Free PMC article.
-
Macrophage Origin, Metabolic Reprogramming and IL-1 Signaling: Promises and Pitfalls in Lung Cancer.Cancers (Basel). 2019 Mar 2;11(3):298. doi: 10.3390/cancers11030298. Cancers (Basel). 2019. PMID: 30832375 Free PMC article. Review.
-
Blockade of SIRPα-CD47 axis by anti-SIRPα antibody enhances anti-tumor activity of DXd antibody-drug conjugates.PLoS One. 2024 Jun 6;19(6):e0304985. doi: 10.1371/journal.pone.0304985. eCollection 2024. PLoS One. 2024. PMID: 38843278 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

