Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity
- PMID: 29144460
- PMCID: PMC5884449
- DOI: 10.1038/nature24302
Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity
Erratum in
-
Erratum: Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity.Nature. 2017 Dec 21;552(7685):430. doi: 10.1038/nature25028. Epub 2017 Nov 22. Nature. 2017. PMID: 29168501 Free PMC article.
-
Author Correction: Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity.Nature. 2018 Sep;561(7721):E1. doi: 10.1038/s41586-018-0304-y. Nature. 2018. PMID: 29973714 Free PMC article.
Abstract
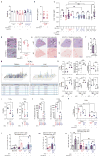
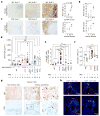
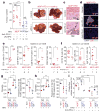
The role of adaptive immunity in early cancer development is controversial. Here we show that chronic inflammation and fibrosis in humans and mice with non-alcoholic fatty liver disease is accompanied by accumulation of liver-resident immunoglobulin-A-producing (IgA+) cells. These cells also express programmed death ligand 1 (PD-L1) and interleukin-10, and directly suppress liver cytotoxic CD8+ T lymphocytes, which prevent emergence of hepatocellular carcinoma and express a limited repertoire of T-cell receptors against tumour-associated antigens. Whereas CD8+ T-cell ablation accelerates hepatocellular carcinoma, genetic or pharmacological interference with IgA+ cell generation attenuates liver carcinogenesis and induces cytotoxic T-lymphocyte-mediated regression of established hepatocellular carcinoma. These findings establish the importance of inflammation-induced suppression of cytotoxic CD8+ T-lymphocyte activation as a tumour-promoting mechanism.
Conflict of interest statement
The authors declare no competing financial interests.
Figures











Comment in
-
Liver cancer: A complex interplay between inflammation and immunity in liver cancer.Nat Rev Gastroenterol Hepatol. 2018 Jan;15(1):3. doi: 10.1038/nrgastro.2017.165. Epub 2017 Nov 22. Nat Rev Gastroenterol Hepatol. 2018. PMID: 29162936 No abstract available.
-
The ABC of adaptive immunity in liver cancer.Hepatology. 2018 Aug;68(2):777-779. doi: 10.1002/hep.29839. Epub 2018 May 14. Hepatology. 2018. PMID: 29427558 Free PMC article. No abstract available.
Similar articles
-
NASH limits anti-tumour surveillance in immunotherapy-treated HCC.Nature. 2021 Apr;592(7854):450-456. doi: 10.1038/s41586-021-03362-0. Epub 2021 Mar 24. Nature. 2021. PMID: 33762733 Free PMC article.
-
Liver fibrosis promotes immune escape in hepatocellular carcinoma via GOLM1-mediated PD-L1 upregulation.Cancer Lett. 2021 Aug 10;513:14-25. doi: 10.1016/j.canlet.2021.05.007. Epub 2021 May 14. Cancer Lett. 2021. PMID: 33992711
-
[Non alcoholic-fatty liver disease causes selective CD4+ T lymphocytes loss and promotes hepatocarcinogenesis].Med Sci (Paris). 2016 Nov;32(11):1023-1026. doi: 10.1051/medsci/20163211021. Epub 2016 Dec 23. Med Sci (Paris). 2016. PMID: 28008846 French. No abstract available.
-
Neutrophil: An emerging player in the occurrence and progression of metabolic associated fatty liver disease.Int Immunopharmacol. 2021 Aug;97:107609. doi: 10.1016/j.intimp.2021.107609. Epub 2021 Apr 19. Int Immunopharmacol. 2021. PMID: 33887577 Review.
-
Adaptive immunity: an emerging player in the progression of NAFLD.Nat Rev Gastroenterol Hepatol. 2020 Feb;17(2):81-92. doi: 10.1038/s41575-019-0210-2. Epub 2019 Oct 11. Nat Rev Gastroenterol Hepatol. 2020. PMID: 31605031 Free PMC article. Review.
Cited by
-
Chinese herbal formula in the treatment of metabolic dysfunction-associated steatotic liver disease: current evidence and practice.Front Med (Lausanne). 2024 Oct 8;11:1476419. doi: 10.3389/fmed.2024.1476419. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39440040 Free PMC article. Review.
-
The multifaceted roles of B lymphocytes in metabolic dysfunction-associated steatotic liver disease.Front Immunol. 2024 Sep 20;15:1447391. doi: 10.3389/fimmu.2024.1447391. eCollection 2024. Front Immunol. 2024. PMID: 39372417 Free PMC article. Review.
-
Macrophages and T cells in metabolic disorder-associated cancers.Nat Rev Cancer. 2024 Nov;24(11):744-767. doi: 10.1038/s41568-024-00743-1. Epub 2024 Oct 1. Nat Rev Cancer. 2024. PMID: 39354070 Review.
-
B-cell-specific signatures reveal novel immunophenotyping and therapeutic targets for hepatocellular carcinoma.World J Gastroenterol. 2024 Sep 14;30(34):3894-3925. doi: 10.3748/wjg.v30.i34.3894. World J Gastroenterol. 2024. PMID: 39350784 Free PMC article.
-
Targeted therapies in hepatocellular carcinoma: past, present, and future.Front Oncol. 2024 Aug 29;14:1432423. doi: 10.3389/fonc.2024.1432423. eCollection 2024. Front Oncol. 2024. PMID: 39267840 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL088093/HL/NHLBI NIH HHS/United States
- GMS10RR029121/NH/NIH HHS/United States
- S10 RR029121/RR/NCRR NIH HHS/United States
- P01 CA128814/CA/NCI NIH HHS/United States
- R37 AI043477/AI/NIAID NIH HHS/United States
- P41 GM103484/GM/NIGMS NIH HHS/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- AI043477/NH/NIH HHS/United States
- DK106419/NH/NIH HHS/United States
- P41GM103484-07/NH/NIH HHS/United States
- R01 CA118165/CA/NCI NIH HHS/United States
- U01 AA022614/AA/NIAAA NIH HHS/United States
- R01 AI043477/AI/NIAID NIH HHS/United States
- CA118165/NH/NIH HHS/United States
- R01 DK106419/DK/NIDDK NIH HHS/United States
- R01 CA127923/CA/NCI NIH HHS/United States
- MR/N005872/1/MRC_/Medical Research Council/United Kingdom
- R01 CA198103/CA/NCI NIH HHS/United States
- CA127923/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

