Epstein-Barr Virus Hijacks DNA Damage Response Transducers to Orchestrate Its Life Cycle
- PMID: 29144413
- PMCID: PMC5707548
- DOI: 10.3390/v9110341
Epstein-Barr Virus Hijacks DNA Damage Response Transducers to Orchestrate Its Life Cycle
Abstract
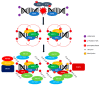
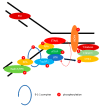
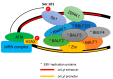
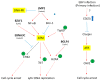
The Epstein-Barr virus (EBV) is a ubiquitous virus that infects most of the human population. EBV infection is associated with multiple human cancers, including Burkitt's lymphoma, Hodgkin's lymphoma, a subset of gastric carcinomas, and almost all undifferentiated non-keratinizing nasopharyngeal carcinoma. Intensive research has shown that EBV triggers a DNA damage response (DDR) during primary infection and lytic reactivation. The EBV-encoded viral proteins have been implicated in deregulating the DDR signaling pathways. The consequences of DDR inactivation lead to genomic instability and promote cellular transformation. This review summarizes the current understanding of the relationship between EBV infection and the DDR transducers, including ATM (ataxia telangiectasia mutated), ATR (ATM and Rad3-related), and DNA-PK (DNA-dependent protein kinase), and discusses how EBV manipulates the DDR signaling pathways to complete the replication process of viral DNA during lytic reactivation.
Keywords: DNA damage response; Epstein–Barr virus; lytic reactivation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
miR-18a reactivates the Epstein-Barr virus through defective DNA damage response and promotes genomic instability in EBV-associated lymphomas.BMC Cancer. 2018 Dec 29;18(1):1293. doi: 10.1186/s12885-018-5205-9. BMC Cancer. 2018. PMID: 30594162 Free PMC article.
-
DNA Damage Signaling Is Induced in the Absence of Epstein-Barr Virus (EBV) Lytic DNA Replication and in Response to Expression of ZEBRA.PLoS One. 2015 May 7;10(5):e0126088. doi: 10.1371/journal.pone.0126088. eCollection 2015. PLoS One. 2015. PMID: 25950714 Free PMC article.
-
Interferon-γ-inducible protein 16 (IFI16) is required for the maintenance of Epstein-Barr virus latency.Virol J. 2017 Nov 13;14(1):221. doi: 10.1186/s12985-017-0891-5. Virol J. 2017. PMID: 29132393 Free PMC article.
-
Epstein-Barr Virus (EBV)-Related Lymphoproliferative Disorders in Ataxia Telangiectasia: Does ATM Regulate EBV Life Cycle?Front Immunol. 2019 Jan 4;9:3060. doi: 10.3389/fimmu.2018.03060. eCollection 2018. Front Immunol. 2019. PMID: 30662441 Free PMC article. Review.
-
Regulation of the latent-lytic switch in Epstein-Barr virus.Semin Cancer Biol. 2014 Jun;26:60-8. doi: 10.1016/j.semcancer.2014.01.002. Epub 2014 Jan 20. Semin Cancer Biol. 2014. PMID: 24457012 Free PMC article. Review.
Cited by
-
Evasion of Intracellular DNA Sensing by Human Herpesviruses.Front Cell Infect Microbiol. 2021 Mar 15;11:647992. doi: 10.3389/fcimb.2021.647992. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33791247 Free PMC article. Review.
-
Rewiring of B cell receptor signaling by Epstein-Barr virus LMP2A.Proc Natl Acad Sci U S A. 2020 Oct 20;117(42):26318-26327. doi: 10.1073/pnas.2007946117. Epub 2020 Oct 5. Proc Natl Acad Sci U S A. 2020. PMID: 33020271 Free PMC article.
-
Evidence for a Hepatitis B Virus Short RNA Fragment Directly Targeting the Cellular RRM2 Gene.Cells. 2022 Jul 20;11(14):2248. doi: 10.3390/cells11142248. Cells. 2022. PMID: 35883690 Free PMC article.
-
Hallmarks of Cancers: Primary Antibody Deficiency Versus Other Inborn Errors of Immunity.Front Immunol. 2021 Aug 17;12:720025. doi: 10.3389/fimmu.2021.720025. eCollection 2021. Front Immunol. 2021. PMID: 34484227 Free PMC article. Review.
-
Immune Modulation by Epstein-Barr Virus Lytic Cycle: Relevance and Implication in Oncogenesis.Pathogens. 2024 Oct 8;13(10):876. doi: 10.3390/pathogens13100876. Pathogens. 2024. PMID: 39452747 Free PMC article. Review.
References
-
- Epstein A. Why and how epstein-barr virus was discovered 50 years ago. Curr. Top. Microbiol. Immunol. 2015;390:3–15. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

