Single-Dose Trivalent VesiculoVax Vaccine Protects Macaques from Lethal Ebolavirus and Marburgvirus Challenge
- PMID: 29142131
- PMCID: PMC5774882
- DOI: 10.1128/JVI.01190-17
Single-Dose Trivalent VesiculoVax Vaccine Protects Macaques from Lethal Ebolavirus and Marburgvirus Challenge
Abstract
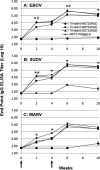
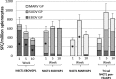
Previous studies demonstrated that a single intramuscular (i.m.) dose of an attenuated recombinant vesicular stomatitis virus (rVSV) vector (VesiculoVax vector platform; rVSV-N4CT1) expressing the glycoprotein (GP) from the Mayinga strain of Zaire ebolavirus (EBOV) protected nonhuman primates (NHPs) from lethal challenge with EBOV strains Kikwit and Makona. Here, we studied the immunogenicities of an expanded range of attenuated rVSV vectors expressing filovirus GP in mice. Based on data from those studies, an optimal attenuated trivalent rVSV vector formulation was identified that included rVSV vectors expressing EBOV, Sudan ebolavirus (SUDV), and the Angola strain of Marburg marburgvirus (MARV) GPs. NHPs were vaccinated with a single dose of the trivalent formulation, followed by lethal challenge 28 days later with each of the three corresponding filoviruses. At day 14 postvaccination, a serum IgG response specific for all three GPs was detected in all the vaccinated macaques. A modest and balanced cell-mediated immune response specific for each GP was also detected in a majority of the vaccinated macaques. No matter the level of total GP-specific immune response detected postvaccination, all the vaccinated macaques were protected from disease and death following lethal challenge with each of the three filoviruses. These findings indicate that vaccination with a single dose of attenuated rVSV-N4CT1 vectors each expressing a single filovirus GP may provide protection against the filoviruses most commonly responsible for outbreaks of hemorrhagic fever in sub-Saharan Africa.IMPORTANCE The West African Ebola virus Zaire outbreak in 2013 showed that the disease was not only a regional concern, but a worldwide problem, and highlighted the need for a safe and efficacious vaccine to be administered to the populace. However, other endemic pathogens, like Ebola virus Sudan and Marburg, also pose an important health risk to the public and therefore require development of a vaccine prior to the occurrence of an outbreak. The significance of our research was the development of a blended trivalent filovirus vaccine that elicited a balanced immune response when administered as a single dose and provided complete protection against a lethal challenge with all three filovirus pathogens.
Keywords: Ebola virus vaccine; Marburg virus vaccine; attenuation; challenge; glycoprotein; nonhuman primates; protection; rVSV vector; trivalent; vaccine.
Copyright © 2018 American Society for Microbiology.
Figures

Similar articles
-
Vaccination With a Highly Attenuated Recombinant Vesicular Stomatitis Virus Vector Protects Against Challenge With a Lethal Dose of Ebola Virus.J Infect Dis. 2015 Oct 1;212 Suppl 2(Suppl 2):S443-51. doi: 10.1093/infdis/jiv316. Epub 2015 Jun 24. J Infect Dis. 2015. PMID: 26109675 Free PMC article.
-
Recombinant Protein Filovirus Vaccines Protect Cynomolgus Macaques From Ebola, Sudan, and Marburg Viruses.Front Immunol. 2021 Aug 18;12:703986. doi: 10.3389/fimmu.2021.703986. eCollection 2021. Front Immunol. 2021. PMID: 34484200 Free PMC article.
-
A highly attenuated Vesiculovax vaccine rapidly protects nonhuman primates against lethal Marburg virus challenge.PLoS Negl Trop Dis. 2022 May 27;16(5):e0010433. doi: 10.1371/journal.pntd.0010433. eCollection 2022 May. PLoS Negl Trop Dis. 2022. PMID: 35622847 Free PMC article.
-
Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections.J Infect Dis. 2011 Nov;204 Suppl 3(Suppl 3):S1075-81. doi: 10.1093/infdis/jir349. J Infect Dis. 2011. PMID: 21987744 Free PMC article. Review.
-
Prospects for immunisation against Marburg and Ebola viruses.Rev Med Virol. 2010 Nov;20(6):344-57. doi: 10.1002/rmv.661. Rev Med Virol. 2010. PMID: 20658513 Free PMC article. Review.
Cited by
-
Efficacy and Immunogenicity of a Recombinant Vesicular Stomatitis Virus-Vectored Marburg Vaccine in Cynomolgus Macaques.Viruses. 2024 Jul 24;16(8):1181. doi: 10.3390/v16081181. Viruses. 2024. PMID: 39205155 Free PMC article.
-
Hemorrhagic Fever-Causing Arenaviruses: Lethal Pathogens and Potent Immune Suppressors.Front Immunol. 2019 Mar 13;10:372. doi: 10.3389/fimmu.2019.00372. eCollection 2019. Front Immunol. 2019. PMID: 30918506 Free PMC article. Review.
-
Design of universal Ebola virus vaccine candidates via immunofocusing.bioRxiv [Preprint]. 2023 Oct 17:2023.10.14.562364. doi: 10.1101/2023.10.14.562364. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2024 Feb 13;121(7):e2316960121. doi: 10.1073/pnas.2316960121 PMID: 37904982 Free PMC article. Updated. Preprint.
-
Filovirus vaccines as a response paradigm for emerging infectious diseases.NPJ Vaccines. 2024 Oct 11;9(1):186. doi: 10.1038/s41541-024-00985-y. NPJ Vaccines. 2024. PMID: 39394249 Free PMC article. Review.
-
Inducing broad-based immunity against viruses with pandemic potential.Immunity. 2022 May 10;55(5):738-748. doi: 10.1016/j.immuni.2022.04.010. Immunity. 2022. PMID: 35545026 Free PMC article. Review.
References
-
- Feldmann H, Sanchez A, Geisbert T. 2013. Filoviridae: Marburg and Ebola viruses, p 923–956. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed (electronic) Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Kuhn JH, Becker S, Ebihara H, Geisbert TW, Johnson KM, Kawaoka Y, Lipkin WI, Negredo AI, Netesov SV, Nichol ST, Palacios G, Peters CJ, Tenorio A, Volchkov VE, Jahrling PB. 2010. Proposal for a revised taxonomy of the family Filoviridae: classification, names of taxa and viruses, and virus abbreviations. Arch Virol 155:2083–2103. doi:10.1007/s00705-010-0814-x. - DOI - PMC - PubMed
-
- Johnson ED, Johnson BK, Silverstein D, Tukei P, Geisbert TW, Sanchez AN, Jahrling PB. 1996. Characterization of a new Marburg virus isolated from a 1987 fatal case in Kenya. Arch Virol Suppl 11:101–114. - PubMed
-
- Negredo A, Palacios G, Vazquez-Moron S, Gonzalez F, Dopazo H, Molero F, Juste J, Quetglas J, Savji N, de la Cruz Martinez M, Herrera JE, Pizarro M, Hutchison SK, Echevarria JE, Lipkin WI, Tenorio A. 2011. Discovery of an ebolavirus-like filovirus in Europe. PLoS Pathog 7:e1002304. doi:10.1371/journal.ppat.1002304. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

