The Mammalian Circadian Clock Exhibits Chronic Ethanol Tolerance and Withdrawal-Induced Glutamate Hypersensitivity, Accompanied by Changes in Glutamate and TrkB Receptor Proteins
- PMID: 29139560
- PMCID: PMC5785434
- DOI: 10.1111/acer.13554
The Mammalian Circadian Clock Exhibits Chronic Ethanol Tolerance and Withdrawal-Induced Glutamate Hypersensitivity, Accompanied by Changes in Glutamate and TrkB Receptor Proteins
Abstract
Background: Alcohol tolerance and withdrawal-induced effects are criteria for alcohol use disorders listed by the DSM-V. Although tolerance and withdrawal have been studied over many decades, there is still uncertainty regarding mechanistic distinctions that characterize these different forms of ethanol (EtOH)-induced plasticity. Previously, we demonstrated that the suprachiasmatic nucleus (SCN) circadian clock develops both acute and rapid tolerance to EtOH inhibition of glutamate-induced circadian phase shifts. Here, we demonstrate that chronic EtOH tolerance and withdrawal-induced glutamate hypersensitivity occur in vitro and that rapid tolerance, chronic tolerance, and glutamate hypersensitivity have distinct cellular changes.
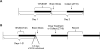
Methods: We use single-unit extracellular electrophysiological recordings to determine whether chronic tolerance to EtOH inhibition of glutamatergic phase shifts and withdrawal-induced glutamate hypersensitivity develop in the SCN. We use Western blotting to compare phosphorylation state and total expression of N-methyl-D-aspartate (NMDA) receptor subunits and associated proteins in the SCN after mice were exposed to varying EtOH consumption paradigms.
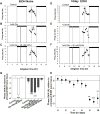
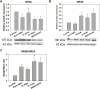
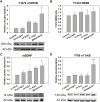
Results: Chronic tolerance developed after a minimum of 8 days of 4 h/d EtOH access, as indicated by a decreased sensitivity to EtOH inhibition of glutamate-induced phase shifts. We also observed an increased sensitivity to glutamate-induced phase shifts in SCN tissue following withdrawal. We demonstrated an increase in the ratio of NR2B:NR2A NMDA receptor subunit expression after 21 days, but not after 10 days of EtOH drinking. This increase persisted during EtOH withdrawal, along with an increase in NR2B Y1472 phosphorylation, mature brain-derived neurotrophic factor, and phosphorylated TrkB.
Conclusions: These results demonstrate that multiple tolerance forms and withdrawal-induced glutamate hypersensitivity occur in the SCN and that these different forms of EtOH-induced plasticity are accompanied by distinct changes in cellular physiology. Importantly, this study further demonstrates the power of using the SCN as a model system to investigate EtOH-induced plasticity.
Keywords: Brain-Derived Neurotrophic Factor; Circadian Rhythms; NMDA; Tolerance; TrkB; Withdrawal.
Copyright © 2017 by the Research Society on Alcoholism.
Figures

Similar articles
-
The mammalian circadian clock in the suprachiasmatic nucleus exhibits rapid tolerance to ethanol in vivo and in vitro.Alcohol Clin Exp Res. 2014 Mar;38(3):760-9. doi: 10.1111/acer.12303. Epub 2014 Feb 11. Alcohol Clin Exp Res. 2014. PMID: 24512529
-
Copper chelation and exogenous copper affect circadian clock phase resetting in the suprachiasmatic nucleus in vitro.Neuroscience. 2014 Jan 3;256:252-61. doi: 10.1016/j.neuroscience.2013.10.033. Epub 2013 Oct 23. Neuroscience. 2014. PMID: 24161278
-
The mammalian circadian clock exhibits acute tolerance to ethanol.Alcohol Clin Exp Res. 2009 Dec;33(12):2088-93. doi: 10.1111/j.1530-0277.2009.01048.x. Epub 2009 Sep 9. Alcohol Clin Exp Res. 2009. PMID: 19740133 Free PMC article.
-
Assessing ethanol's actions in the suprachiasmatic circadian clock using in vivo and in vitro approaches.Alcohol. 2015 Jun;49(4):321-339. doi: 10.1016/j.alcohol.2014.07.016. Epub 2014 Oct 18. Alcohol. 2015. PMID: 25457753 Free PMC article. Review.
-
Role of polyamines and NMDA receptors in ethanol dependence and withdrawal.Alcohol Clin Exp Res. 2001 May;25(5 Suppl ISBRA):132S-136S. doi: 10.1097/00000374-200105051-00023. Alcohol Clin Exp Res. 2001. PMID: 11391062 Review.
Cited by
-
Alcohol tolerance encoding in sleep regulatory circadian neurons in Drosophila.bioRxiv [Preprint]. 2023 Feb 2:2023.01.30.526363. doi: 10.1101/2023.01.30.526363. bioRxiv. 2023. Update in: Addict Biol. 2023 Aug;28(8):e13304. doi: 10.1111/adb.13304. PMID: 36778487 Free PMC article. Updated. Preprint.
-
Alcohol sensitivity and tolerance encoding in sleep regulatory circadian neurons in Drosophila.Addict Biol. 2023 Aug;28(8):e13304. doi: 10.1111/adb.13304. Addict Biol. 2023. PMID: 37500483 Free PMC article.
References
-
- Beckley JT, Laguesse S, Phamluong K, Morisot N, Wegner SA, Ron D. The first alcohol drink triggers mTORC1-dependent synaptic plasticity in nucleus accumbens dopamine d1 receptor neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2016 Jan;36(3):701–713. - PMC - PubMed
-
- Bell RL, Stewart RB, Woods JE, Lumeng L, Li TK, Murphy JM, McBride WJ. Responsivity and development of tolerance to the motor impairing effects of moderate doses of ethanol in alcohol-preferring (p) and -nonpreferring (NP) rat lines. Alcoholism, clinical and experimental research. 2001 May;25(5):644–650. - PubMed
-
- Bradbury MJ, Dement WC, Edgar DM. Serotonin-containing fibers in the suprachiasmatic hypothalamus attenuate light-induced phase delays in mice. Brain Research. 1997 Sep;768(1–2):125–134. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

