The M25 gene products are critical for the cytopathic effect of mouse cytomegalovirus
- PMID: 29138436
- PMCID: PMC5686157
- DOI: 10.1038/s41598-017-15783-x
The M25 gene products are critical for the cytopathic effect of mouse cytomegalovirus
Abstract
Cell rounding is a hallmark of the cytopathic effect induced by cytomegaloviruses. By screening a panel of deletion mutants of mouse cytomegalovirus (MCMV) a mutant was identified that did not elicit cell rounding and lacked the ability to form typical plaques. Altered cell morphology was assigned to the viral M25 gene. We detected an early 2.8 kb M25 mRNA directing the synthesis of a 105 kDa M25 protein, and confirmed that a late 3.1 kb mRNA encodes a 130 kDa M25 tegument protein. Virions lacking the M25 tegument protein were of smaller size because the tegument layer between capsid and viral envelope was reduced. The ΔM25 mutant did not provoke the rearrangement of the actin cytoskeleton observed after wild-type MCMV infection, and isolated expression of the M25 proteins led to cell size reduction, confirming that they contribute to the morphological changes. Yields of progeny virus and cell-to-cell spread of the ΔM25 mutant in vitro were diminished and replication in vivo was impaired. The identification of an MCMV gene involved in cell rounding provides the basis for investigating the role of this cytopathic effect in CMV pathogenesis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
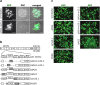
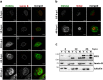
 , kanamycin resistance gene; ---, deleted sequences; → , orientation of ORFs; ▪, FRT sites. (c) Cells infected with the indicated viruses were examined by fluorescence microscopy 48 h p.i. Scale bars, 100 µm.
, kanamycin resistance gene; ---, deleted sequences; → , orientation of ORFs; ▪, FRT sites. (c) Cells infected with the indicated viruses were examined by fluorescence microscopy 48 h p.i. Scale bars, 100 µm.

Similar articles
-
Characterization of M116.1p, a Murine Cytomegalovirus Protein Required for Efficient Infection of Mononuclear Phagocytes.J Virol. 2022 Jan 26;96(2):e0087621. doi: 10.1128/JVI.00876-21. Epub 2021 Oct 27. J Virol. 2022. PMID: 34705561 Free PMC article.
-
Murine Cytomegalovirus M25 Proteins Sequester the Tumor Suppressor Protein p53 in Nuclear Accumulations.J Virol. 2020 Sep 29;94(20):e00574-20. doi: 10.1128/JVI.00574-20. Print 2020 Sep 29. J Virol. 2020. PMID: 32727874 Free PMC article.
-
The murine cytomegalovirus M25 open reading frame encodes a component of the tegument.Virology. 1999 Sep 30;262(2):265-76. doi: 10.1006/viro.1999.9942. Virology. 1999. PMID: 10502507
-
Genetic analyses of gene function and pathogenesis of murine cytomegalovirus by transposon-mediated mutagenesis.J Clin Virol. 2002 Aug;25 Suppl 2:S111-22. doi: 10.1016/s1386-6532(02)00096-3. J Clin Virol. 2002. PMID: 12361762 Review.
-
Viral determinants influencing intra- and intercellular communication in cytomegalovirus infection.Curr Opin Virol. 2023 Jun;60:101328. doi: 10.1016/j.coviro.2023.101328. Epub 2023 Apr 7. Curr Opin Virol. 2023. PMID: 37031486 Review.
Cited by
-
Dynamin Inhibitors Prevent the Establishment of the Cytomegalovirus Assembly Compartment in the Early Phase of Infection.Life (Basel). 2021 Aug 25;11(9):876. doi: 10.3390/life11090876. Life (Basel). 2021. PMID: 34575026 Free PMC article.
-
Cytomegalovirus Generates Assembly Compartment in the Early Phase of Infection by Perturbation of Host-Cell Factors Recruitment at the Early Endosome/Endosomal Recycling Compartment/Trans-Golgi Interface.Front Cell Dev Biol. 2020 Sep 11;8:563607. doi: 10.3389/fcell.2020.563607. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33042998 Free PMC article.
-
Characterization of M116.1p, a Murine Cytomegalovirus Protein Required for Efficient Infection of Mononuclear Phagocytes.J Virol. 2022 Jan 26;96(2):e0087621. doi: 10.1128/JVI.00876-21. Epub 2021 Oct 27. J Virol. 2022. PMID: 34705561 Free PMC article.
-
The Abundant Tegument Protein pUL25 of Human Cytomegalovirus Prevents Proteasomal Degradation of pUL26 and Supports Its Suppression of ISGylation.J Virol. 2018 Nov 27;92(24):e01180-18. doi: 10.1128/JVI.01180-18. Print 2018 Dec 15. J Virol. 2018. PMID: 30282718 Free PMC article.
-
Immunometabolic phenotype of BV-2 microglia cells upon murine cytomegalovirus infection.J Neurovirol. 2019 Aug;25(4):496-507. doi: 10.1007/s13365-019-00750-1. Epub 2019 Apr 25. J Neurovirol. 2019. PMID: 31025265
References
-
- Condit, R. C. Principles of Virology in Fields Virology (eds Knipe, D. M. & Howley, P. M.) 21–51 (Lippincott Williams & Wilkins, 2013).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

