Whole Exome Sequencing of Patients with Steroid-Resistant Nephrotic Syndrome
- PMID: 29127259
- PMCID: PMC5753307
- DOI: 10.2215/CJN.04120417
Whole Exome Sequencing of Patients with Steroid-Resistant Nephrotic Syndrome
Abstract
Background and objectives: Steroid-resistant nephrotic syndrome overwhelmingly progresses to ESRD. More than 30 monogenic genes have been identified to cause steroid-resistant nephrotic syndrome. We previously detected causative mutations using targeted panel sequencing in 30% of patients with steroid-resistant nephrotic syndrome. Panel sequencing has a number of limitations when compared with whole exome sequencing. We employed whole exome sequencing to detect monogenic causes of steroid-resistant nephrotic syndrome in an international cohort of 300 families.
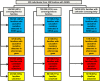
Design, setting, participants, & measurements: Three hundred thirty-five individuals with steroid-resistant nephrotic syndrome from 300 families were recruited from April of 1998 to June of 2016. Age of onset was restricted to <25 years of age. Exome data were evaluated for 33 known monogenic steroid-resistant nephrotic syndrome genes.
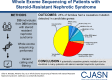
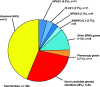
Results: In 74 of 300 families (25%), we identified a causative mutation in one of 20 genes known to cause steroid-resistant nephrotic syndrome. In 11 families (3.7%), we detected a mutation in a gene that causes a phenocopy of steroid-resistant nephrotic syndrome. This is consistent with our previously published identification of mutations using a panel approach. We detected a causative mutation in a known steroid-resistant nephrotic syndrome gene in 38% of consanguineous families and in 13% of nonconsanguineous families, and 48% of children with congenital nephrotic syndrome. A total of 68 different mutations were detected in 20 of 33 steroid-resistant nephrotic syndrome genes. Fifteen of these mutations were novel. NPHS1, PLCE1, NPHS2, and SMARCAL1 were the most common genes in which we detected a mutation. In another 28% of families, we detected mutations in one or more candidate genes for steroid-resistant nephrotic syndrome.
Conclusions: Whole exome sequencing is a sensitive approach toward diagnosis of monogenic causes of steroid-resistant nephrotic syndrome. A molecular genetic diagnosis of steroid-resistant nephrotic syndrome may have important consequences for the management of treatment and kidney transplantation in steroid-resistant nephrotic syndrome.
Keywords: Child; Exome; Humans; Kidney Failure, Chronic; Mutation; Nephrosis, congenital; Phenotype; Renal Insufficiency, Chronic; genetic renal disease; kidney transplantation; molecular genetics; nephrotic syndrome; pediatric.
Copyright © 2018 by the American Society of Nephrology.
Figures


Similar articles
-
Genomic and clinical profiling of a national nephrotic syndrome cohort advocates a precision medicine approach to disease management.Kidney Int. 2017 Apr;91(4):937-947. doi: 10.1016/j.kint.2016.10.013. Epub 2017 Jan 20. Kidney Int. 2017. PMID: 28117080
-
Spectrum of steroid-resistant and congenital nephrotic syndrome in children: the PodoNet registry cohort.Clin J Am Soc Nephrol. 2015 Apr 7;10(4):592-600. doi: 10.2215/CJN.06260614. Epub 2015 Jan 29. Clin J Am Soc Nephrol. 2015. PMID: 25635037 Free PMC article.
-
Panel sequencing distinguishes monogenic forms of nephritis from nephrosis in children.Nephrol Dial Transplant. 2019 Mar 1;34(3):474-485. doi: 10.1093/ndt/gfy050. Nephrol Dial Transplant. 2019. PMID: 30295827 Free PMC article.
-
Steroid Resistant Nephrotic Syndrome-Genetic Consideration.Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2015;36(3):5-12. doi: 10.1515/prilozi-2015-0073. Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2015. PMID: 27442391 Review.
-
Hereditary nephrotic syndrome: a systematic approach for genetic testing and a review of associated podocyte gene mutations.Pediatr Nephrol. 2010 Sep;25(9):1621-32. doi: 10.1007/s00467-010-1495-0. Epub 2010 Mar 24. Pediatr Nephrol. 2010. PMID: 20333530 Free PMC article. Review.
Cited by
-
The contributions of deleterious rare alleles in NLRP12 and inflammasome-related genes to polymyalgia rheumatica.Sci Rep. 2024 Jan 4;14(1):490. doi: 10.1038/s41598-024-51320-3. Sci Rep. 2024. PMID: 38177227 Free PMC article.
-
Spectrum of NPHS1 and NPHS2 variants in egyptian children with focal segmental glomerular sclerosis: identification of six novel variants and founder effect.Mol Genet Genomics. 2022 May;297(3):689-698. doi: 10.1007/s00438-022-01877-3. Epub 2022 Mar 12. Mol Genet Genomics. 2022. PMID: 35278126
-
Role of Deleterious Rare Alleles for Acute-Onset Diffuse Interstitial Lung Disease in Collagen Diseases.Clin Med Insights Circ Respir Pulm Med. 2019 Jul 30;13:1179548419866443. doi: 10.1177/1179548419866443. eCollection 2019. Clin Med Insights Circ Respir Pulm Med. 2019. PMID: 31391785 Free PMC article.
-
Identification of novel mutations and phenotype in the steroid resistant nephrotic syndrome gene NUP93: a case report.BMC Nephrol. 2019 Jul 17;20(1):271. doi: 10.1186/s12882-019-1458-z. BMC Nephrol. 2019. PMID: 31315584 Free PMC article.
-
Assessment of podocyte detachment as a pivotal step in the development of focal segmental glomerulosclerosis.J Egypt Natl Canc Inst. 2024 Nov 18;36(1):36. doi: 10.1186/s43046-024-00244-0. J Egypt Natl Canc Inst. 2024. PMID: 39551885
References
-
- Primary nephrotic syndrome in children: Clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int 20: 765–771, 1981 - PubMed
-
- Smith JM, Stablein DM, Munoz R, Hebert D, McDonald RA: Contributions of the transplant registry: The 2006 annual report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS). Pediatr Transplant 11: 366–373, 2007 - PubMed
-
- Cheong HI, Han HW, Park HW, Ha IS, Han KS, Lee HS, Kim SJ, Choi Y: Early recurrent nephrotic syndrome after renal transplantation in children with focal segmental glomerulosclerosis. Nephrol Dial Transplant 15: 78–81, 2000 - PubMed
-
- Senggutuvan P, Cameron JS, Hartley RB, Rigden S, Chantler C, Haycock G, Williams DG, Ogg C, Koffman G: Recurrence of focal segmental glomerulosclerosis in transplanted kidneys: Analysis of incidence and risk factors in 59 allografts. Pediatr Nephrol 4: 21–28, 1990 - PubMed
-
- Hamiwka LA, Midgley JP, Wade AW, Martz KL, Grisaru S. Outcomes of kidney transplantation in children with nephronophthisis: an analysis of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Registry. Pediatr Transplant 12: 878–882, 2008 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

