Wnt/β‑catenin pathway activation and silencing of the APC gene in HPV‑positive human cervical cancer‑derived cells
- PMID: 29115417
- PMCID: PMC5780127
- DOI: 10.3892/mmr.2017.7853
Wnt/β‑catenin pathway activation and silencing of the APC gene in HPV‑positive human cervical cancer‑derived cells
Abstract
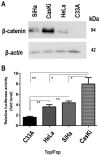
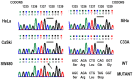
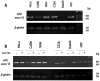
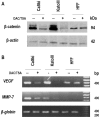
Although persistent infections with high‑risk human papilloma virus (HPV) constitute the most significant cofactor for the development of cervical cancer, they are insufficient on their own. Mutations or epigenetic inactivation of the tumor suppressor adenomatous polyposis coli (APC), the two acting as prominent oncogenic mechanisms in a number of types of cancer, are frequently associated with aberrant activation of the Wnt/β‑catenin pathway. According to these observations, it was hypothesized that APC alteration may lead to β‑catenin deregulation and the abnormal expression of direct targets of the Wnt pathway in HPV‑infected cervical cancer cells. The present study confirmed that the stabilization of β‑catenin correlates with enhanced transcriptional activity of the β‑catenin/T‑cell factor complex in cervical cancer cell lines. Sequence analysis of the 'hot‑spot' in the mutation cluster region did not exhibit genetic alterations that may be associated with APC gene inactivation. In addition, it was identified that there was a good correlation with the hypermethylation status of the APC promoter 1A and the abnormal accumulation of endogenous β‑catenin in cell lines and biopsies infected with HPV16, although not HPV18. Removal of the epigenetic markers led to an increase in APC levels and a reduction of β‑catenin expression in two transcriptional targets of the Wnt pathway: Matrix metalloproteinase‑7 and vascular endothelial growth factor. The present study suggested that the increase in Wnt activity in certain cervical cancer‑derived cells may be associated with an alteration in the methylation status of the APC gene promoter 1A.
Figures

Similar articles
-
Genetic and epigenetic alterations of the APC gene in malignant melanoma.Oncogene. 2004 Jul 1;23(30):5215-26. doi: 10.1038/sj.onc.1207647. Oncogene. 2004. PMID: 15133491
-
High-risk HPV infection modulates the promoter hypermethylation of APC, SFRP1, and PTEN in cervical cancer patients of North India.Mol Biol Rep. 2020 Dec;47(12):9725-9732. doi: 10.1007/s11033-020-05960-z. Epub 2020 Nov 23. Mol Biol Rep. 2020. PMID: 33230782
-
Hypermethylation of adenomatous polyposis coli gene promoter is associated with novel Wnt signaling pathway in gastric adenomas.J Gastroenterol Hepatol. 2012 Oct;27(10):1629-34. doi: 10.1111/j.1440-1746.2012.07219.x. J Gastroenterol Hepatol. 2012. PMID: 22741528
-
Integrative analysis of aberrant Wnt signaling in hepatitis B virus-related hepatocellular carcinoma.World J Gastroenterol. 2015 May 28;21(20):6317-28. doi: 10.3748/wjg.v21.i20.6317. World J Gastroenterol. 2015. PMID: 26034368 Free PMC article. Review.
-
Cribriform morular thyroid carcinoma: Clinicopathological and molecular basis for both a preventive and therapeutic approach for a rare tumor (Review).Oncol Rep. 2024 Sep;52(3):119. doi: 10.3892/or.2024.8778. Epub 2024 Jul 19. Oncol Rep. 2024. PMID: 39027989 Free PMC article. Review.
Cited by
-
Functional New Transcription Factors (TFs) Associated with Cervical Cancer.J Healthc Eng. 2022 Jan 25;2022:8381559. doi: 10.1155/2022/8381559. eCollection 2022. J Healthc Eng. 2022. Retraction in: J Healthc Eng. 2023 Oct 11;2023:9835459. doi: 10.1155/2023/9835459. PMID: 35126951 Free PMC article. Retracted.
-
Epigallocatechin gallate inhibits HeLa cells by modulation of epigenetics and signaling pathways.3 Biotech. 2020 Nov;10(11):484. doi: 10.1007/s13205-020-02473-1. Epub 2020 Oct 23. 3 Biotech. 2020. PMID: 33117625 Free PMC article.
-
Genistein Modulates Signaling Pathways and Targets Several Epigenetic Markers in HeLa Cells.Genes (Basel). 2019 Nov 21;10(12):955. doi: 10.3390/genes10120955. Genes (Basel). 2019. PMID: 31766427 Free PMC article.
-
Gene expression and demographic analyses in women with the poor ovarian response: a computational approach.J Assist Reprod Genet. 2023 Nov;40(11):2627-2638. doi: 10.1007/s10815-023-02919-4. Epub 2023 Aug 29. J Assist Reprod Genet. 2023. PMID: 37642817 Free PMC article.
-
Wnt3a/β-Catenin/CBP Activation in the Progression of Cervical Intraepithelial Neoplasia.Pathol Oncol Res. 2021 Mar 31;27:609620. doi: 10.3389/pore.2021.609620. eCollection 2021. Pathol Oncol Res. 2021. PMID: 34257574 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

