Modulation of Cell Death Pathways by Hepatitis C Virus Proteins in Huh7.5 Hepatoma Cells
- PMID: 29113144
- PMCID: PMC5713315
- DOI: 10.3390/ijms18112346
Modulation of Cell Death Pathways by Hepatitis C Virus Proteins in Huh7.5 Hepatoma Cells
Abstract
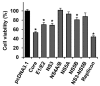
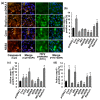
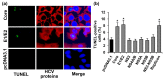
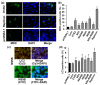
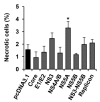
The hepatitis C virus (HCV) causes chronic liver disease leading to fibrosis, cirrhosis, and hepatocellular carcinoma. HCV infection triggers various types of cell death which contribute to hepatitis C pathogenesis. However, much is still unknown about the impact of viral proteins on them. Here we present the results of simultaneous immunocytochemical analysis of markers of apoptosis, autophagy, and necrosis in Huh7.5 cells expressing individual HCV proteins or their combinations, or harboring the virus replicon. Stable replication of the full-length HCV genome or transient expression of its core, Е1/Е2, NS3 and NS5B led to the death of 20-47% cells, 72 h posttransfection, whereas the expression of the NS4A/B, NS5A or NS3-NS5B polyprotein did not affect cell viability. HCV proteins caused different impacts on the activation of caspases-3, -8 and -9 and on DNA fragmentation. The structural core and E1/E2 proteins promoted apoptosis, whereas non-structural NS4A/B, NS5A, NS5B suppressed apoptosis by blocking various members of the caspase cascade. The majority of HCV proteins also enhanced autophagy, while NS5A also induced necrosis. As a result, the death of Huh7.5 cells expressing the HCV core was induced via apoptosis, the cells expressing NS3 and NS5B via autophagy-associated death, and the cells expressing E1/E2 glycoproteins or harboring HCV the replicon via both apoptosis and autophagy.
Keywords: apoptosis; autophagy; caspase; hepatitis C virus; hepatoma Huh7.5 cells; necrosis; replicon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Selectable subgenomic and genome-length dicistronic RNAs derived from an infectious molecular clone of the HCV-N strain of hepatitis C virus replicate efficiently in cultured Huh7 cells.J Virol. 2002 Mar;76(6):2997-3006. doi: 10.1128/jvi.76.6.2997-3006.2002. J Virol. 2002. PMID: 11861865 Free PMC article.
-
Dominant negative effect of wild-type NS5A on NS5A-adapted subgenomic hepatitis C virus RNA replicon.J Gen Virol. 2004 Jul;85(Pt 7):1867-1875. doi: 10.1099/vir.0.80006-0. J Gen Virol. 2004. PMID: 15218171
-
Hepatitis C Virus Proteins Core and NS5A Are Highly Sensitive to Oxidative Stress-Induced Degradation after eIF2α/ATF4 Pathway Activation.Viruses. 2020 Apr 9;12(4):425. doi: 10.3390/v12040425. Viruses. 2020. PMID: 32283772 Free PMC article.
-
Molecular virology of hepatitis C virus.Acta Med Okayama. 2001 Jun;55(3):133-59. doi: 10.18926/AMO/32025. Acta Med Okayama. 2001. PMID: 11434427 Review.
-
Apoptosis in hepatitis C virus infection.Cell Death Differ. 2003 Jan;10 Suppl 1:S48-58. doi: 10.1038/sj.cdd.4401119. Cell Death Differ. 2003. PMID: 12655346 Review.
Cited by
-
FHC, an NS4B-interacting Protein, Enhances Classical Swine Fever Virus Propagation and Acts Positively in Viral Anti-apoptosis.Sci Rep. 2018 May 29;8(1):8318. doi: 10.1038/s41598-018-26777-8. Sci Rep. 2018. PMID: 29844394 Free PMC article.
-
The Role of Bcl-xL Protein in Viral Infections.Int J Mol Sci. 2021 Feb 16;22(4):1956. doi: 10.3390/ijms22041956. Int J Mol Sci. 2021. PMID: 33669408 Free PMC article. Review.
-
The Expression of P35 Plays a Key Role in the Difference in Apoptosis Induced by AcMNPV Infection in Different Spodoptera exigua Cell Lines.Int J Mol Sci. 2023 Aug 25;24(17):13228. doi: 10.3390/ijms241713228. Int J Mol Sci. 2023. PMID: 37686033 Free PMC article.
-
Human Mesenchymal Stem Cells Modified with the NS5A Gene of Hepatitis C Virus Induce a Cellular Immune Response Exceeding the Response to DNA Immunization with This Gene.Biology (Basel). 2023 May 30;12(6):792. doi: 10.3390/biology12060792. Biology (Basel). 2023. PMID: 37372076 Free PMC article.
-
Genetically Modified Mouse Mesenchymal Stem Cells Expressing Non-Structural Proteins of Hepatitis C Virus Induce Effective Immune Response.Vaccines (Basel). 2020 Feb 2;8(1):62. doi: 10.3390/vaccines8010062. Vaccines (Basel). 2020. PMID: 32024236 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

