Investigation of antiviral state mediated by interferon-inducible transmembrane protein 1 induced by H9N2 virus and inactivated viral particle in human endothelial cells
- PMID: 29100522
- PMCID: PMC5670731
- DOI: 10.1186/s12985-017-0875-5
Investigation of antiviral state mediated by interferon-inducible transmembrane protein 1 induced by H9N2 virus and inactivated viral particle in human endothelial cells
Abstract
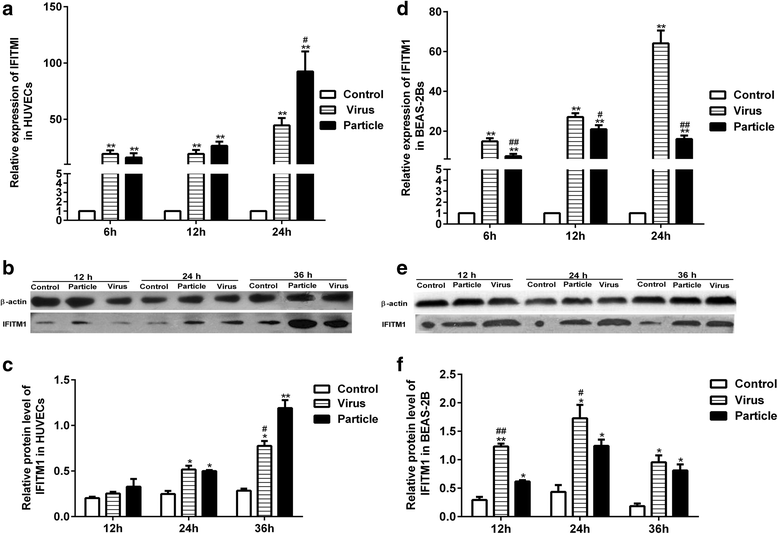
Background: Endothelial cells are believed to play an important role in response to virus infection. Our previous microarray analysis showed that H9N2 virus infection and inactivated viral particle inoculation increased the expression of interferon-inducible transmembrane protein 1 (IFITM1) in human umbilical vein endothelial cells (HUVECs). In present study, we deeply investigated the expression patterns of IFITM1 and IFITM1-mediated antiviral response induced by H9N2 virus infection and inactivated viral particle inoculation in HUVECs. Epithelial cells that are considered target cells of the influenza virus were selected as a reference control.
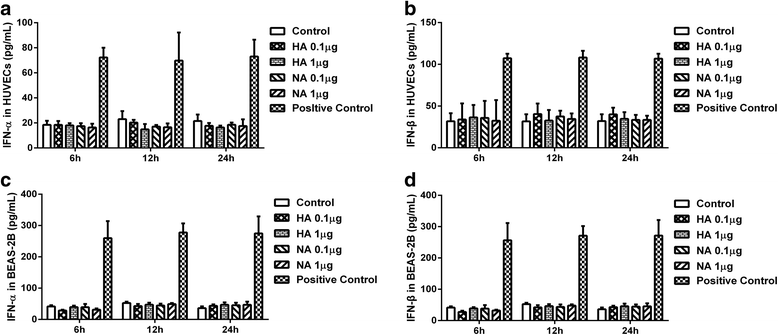
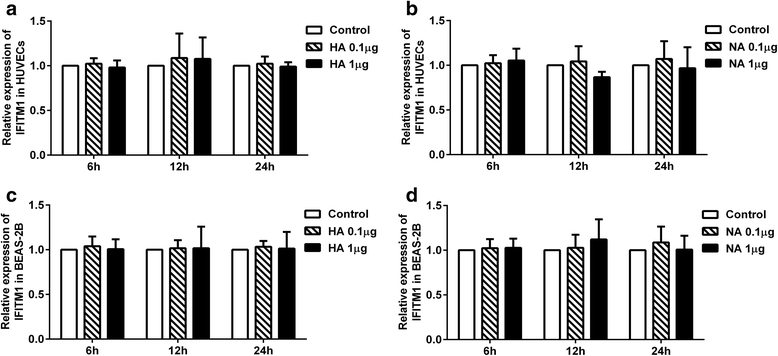
Methods: First, we quantified the expression levels of IFITM1 in HUVECs induced by H9N2 virus infection or viral particle inoculation using quantitative real-time PCR and western blot. Second, we observed whether hemagglutinin or neuraminidase affected IFITM1 expression in HUVECs. Finally, we investigated the effect of induced-IFITM1 on the antiviral state in HUVECs by siRNA and activation plasmid transfection.
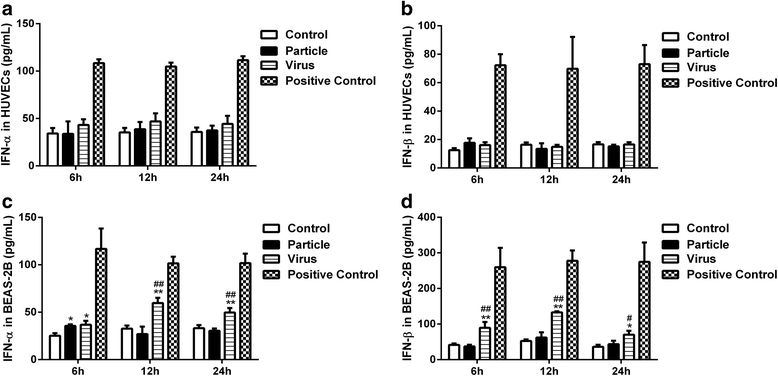
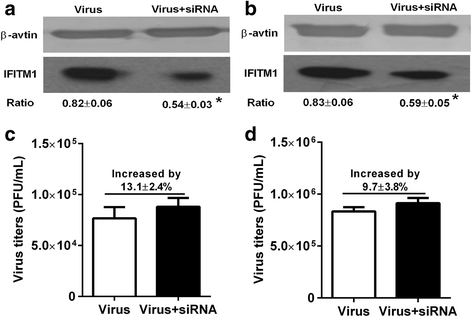
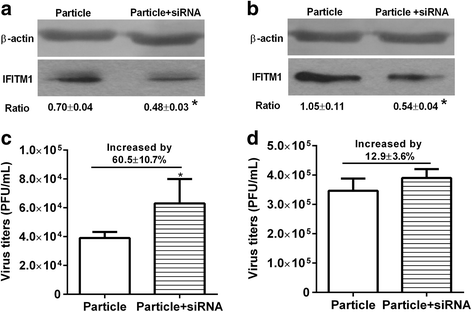
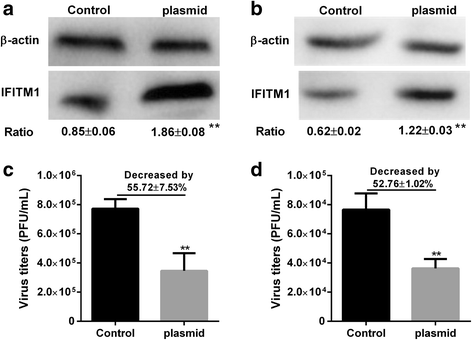
Results: Both H9N2 virus infection and viral particle inoculation increased the expression of IFITM1 without elevating the levels of interferon-ɑ/β in HUVECs. HA or NA protein binding alone is not sufficient to increase the levels of IFITM1 and interferon-ɑ/β in HUVECs. IFITM1 induced by viral particle inoculation significantly decreased the virus titers in culture supernatants of HUVECs.
Conclusions: Our results showed that inactivated viral particle inoculation increased the expression of IFITM1 at mRNA and protein levels. Moreover, the induction of IFITM1 expression mediated the antiviral state in HUVECs.
Keywords: Antiviral state; H9N2 influenza virus; Human endothelial cells; Human epithelial cells; IFITM1; Inactivated viral particle.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
IFIT1 Expression Patterns Induced by H9N2 Virus and Inactivated Viral Particle in Human Umbilical Vein Endothelial Cells and Bronchus Epithelial Cells.Mol Cells. 2018 Apr 30;41(4):271-281. doi: 10.14348/molcells.2018.2091. Epub 2018 Apr 5. Mol Cells. 2018. PMID: 29629559 Free PMC article.
-
Transcriptional response of human umbilical vein endothelial cell to H9N2 influenza virus infection.Virology. 2015 Aug;482:117-27. doi: 10.1016/j.virol.2015.03.037. Epub 2015 Apr 7. Virology. 2015. PMID: 25863179
-
Pre-immune state induced by chicken interferon gamma inhibits the replication of H1N1 human and H9N2 avian influenza viruses in chicken embryo fibroblasts.Virol J. 2016 Apr 27;13:71. doi: 10.1186/s12985-016-0527-1. Virol J. 2016. PMID: 27121613 Free PMC article.
-
Cellular Antiviral Factors that Target Particle Infectivity of HIV-1.Curr HIV Res. 2016;14(3):211-6. doi: 10.2174/1570162x14666151216145521. Curr HIV Res. 2016. PMID: 26674651 Free PMC article. Review.
-
Deciphering the Roles of IFITM1 in Tumors.Mol Diagn Ther. 2020 Aug;24(4):433-441. doi: 10.1007/s40291-020-00469-4. Mol Diagn Ther. 2020. PMID: 32394410 Review.
Cited by
-
Preventing occludin tight-junction disruption via inhibition of microRNA-193b-5p attenuates viral load and influenza-induced lung injury.Mol Ther. 2023 Sep 6;31(9):2681-2701. doi: 10.1016/j.ymthe.2023.06.011. Epub 2023 Jun 19. Mol Ther. 2023. PMID: 37340634 Free PMC article.
-
Chronic exposure to arsenite enhances influenza virus infection in cultured cells.J Appl Toxicol. 2020 Apr;40(4):458-469. doi: 10.1002/jat.3918. Epub 2020 Jan 20. J Appl Toxicol. 2020. PMID: 31960482 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

