Oncogenic driver mutations, treatment, and EGFR-TKI resistance in a Caucasian population with non-small cell lung cancer: survival in clinical practice
- PMID: 29100434
- PMCID: PMC5652823
- DOI: 10.18632/oncotarget.20857
Oncogenic driver mutations, treatment, and EGFR-TKI resistance in a Caucasian population with non-small cell lung cancer: survival in clinical practice
Abstract
Introduction: Oncogenic driver mutations activating EGFR, ALK, or BRAF in NSCLC predict sensitivity to specific tyrosine-kinase inhibitors (TKIs). We provide data on prevalence, treatment and survival of driver-mutation positive NSCLC in a predominantly Caucasian population in routine clinical practice.
Patients and methods: NSCLC patients diagnosed from 2006-2015 with an EGFR-test result were included (n=265). Testing for EGFR, ALK, or BRAF was performed if specific TKI therapy was considered. Case-control analyses of overall survival (OS) comparing driver-mutation positive and negative patients were performed.
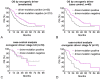
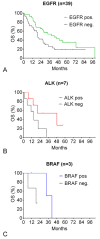
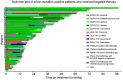
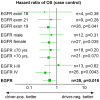
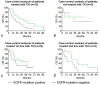
Results: 44 sensitizing EGFR mutations (17%), 8 ALK translocations (7%, n=111) and 3 BRAF mutations (8%, n=39) were detected in adenocarcinoma or adenosquamous carcinoma. We did not find mutations in tumors without an adenocarcinoma-component. More than 90% of inoperable driver-mutation positive patients received TKI-therapy. Case-control analysis revealed improved OS of driver-mutation positive patients (39.6 vs. 19.4 months, HR 0.51). OS was improved in stage IV patients but not in stage I-III patients.OS of EGFR-TKI treated patients was similar for 1st and 2nd-line EGFR-TKI treatment. Patients not treated with EGFR-TKI had no benefit in OS. Re-biopsies obtained at progression revealed an EGFR-T790M mutation in 73% (n=11). These patients responded to the 3rd-generation EGFR-TKI osimertinib.
Discussion: Testing guided by predictive clinical parameters resulted in twice as high rates of mutation-positive patients than expected, and TKI treatment resulted in a strong long-term OS advantage.
Conclusion: Testing for driver mutations is feasible in routine clinical practice, and identifies patients who benefit from TKI-therapy. OS compares favorably with OS in clinical studies.
Keywords: ALK; BRAF; EGFR; NSCLC; overall survival.
Conflict of interest statement
CONFLICTS OF INTEREST None of the authors has declared a conflict of interest.
Figures

Similar articles
-
Observational Study of Treatment Patterns in Patients with Epidermal Growth Factor Receptor (EGFR) Mutation-Positive Non-Small Cell Lung Cancer After First-Line EGFR-Tyrosine Kinase Inhibitors.Adv Ther. 2020 Feb;37(2):946-954. doi: 10.1007/s12325-020-01221-4. Epub 2020 Jan 18. Adv Ther. 2020. PMID: 31955357
-
Management and future directions in non-small cell lung cancer with known activating mutations.Am Soc Clin Oncol Educ Book. 2014:e353-65. doi: 10.14694/EdBook_AM.2014.34.e353. Am Soc Clin Oncol Educ Book. 2014. PMID: 24857124 Review.
-
Picoliter-Droplet Digital Polymerase Chain Reaction-Based Analysis of Cell-Free Plasma DNA to Assess EGFR Mutations in Lung Adenocarcinoma That Confer Resistance to Tyrosine-Kinase Inhibitors.Oncologist. 2016 Feb;21(2):156-64. doi: 10.1634/theoncologist.2015-0288. Epub 2016 Jan 14. Oncologist. 2016. PMID: 26768482 Free PMC article.
-
Clinical features and therapeutic options in non-small cell lung cancer patients with concomitant mutations of EGFR, ALK, ROS1, KRAS or BRAF.Cancer Med. 2019 Jun;8(6):2858-2866. doi: 10.1002/cam4.2183. Epub 2019 Apr 24. Cancer Med. 2019. PMID: 31016879 Free PMC article.
-
Overall Treatment Strategy for Patients With Metastatic NSCLC With Activating EGFR Mutations.Clin Lung Cancer. 2022 Jan;23(1):e69-e82. doi: 10.1016/j.cllc.2021.10.009. Epub 2021 Oct 25. Clin Lung Cancer. 2022. PMID: 34865963 Review.
Cited by
-
PFKFB3 Inhibition Impairs Erlotinib-Induced Autophagy in NSCLCs.Cells. 2021 Jul 3;10(7):1679. doi: 10.3390/cells10071679. Cells. 2021. PMID: 34359849 Free PMC article.
-
Characterization of epidermal growth factor receptor (EGFR) P848L, an unusual EGFR variant present in lung cancer patients, in a murine Ba/F3 model.FEBS Open Bio. 2019 Oct;9(10):1689-1704. doi: 10.1002/2211-5463.12702. Epub 2019 Sep 7. FEBS Open Bio. 2019. PMID: 31314158 Free PMC article.
-
Next-generation sequencing and its clinical application.Cancer Biol Med. 2019 Feb;16(1):4-10. doi: 10.20892/j.issn.2095-3941.2018.0055. Cancer Biol Med. 2019. PMID: 31119042 Free PMC article. No abstract available.
-
Physiologically Based Pharmacokinetic (PBPK) Modeling to Predict PET Image Quality of Three Generations EGFR TKI in Advanced-Stage NSCLC Patients.Pharmaceuticals (Basel). 2022 Jun 27;15(7):796. doi: 10.3390/ph15070796. Pharmaceuticals (Basel). 2022. PMID: 35890095 Free PMC article.
-
Relationship between Biodistribution and Tracer Kinetics of 11C-Erlotinib, 18F-Afatinib and 11C-Osimertinib and Image Quality Evaluation Using Pharmacokinetic/Pharmacodynamic Analysis in Advanced Stage Non-Small Cell Lung Cancer Patients.Diagnostics (Basel). 2022 Apr 1;12(4):883. doi: 10.3390/diagnostics12040883. Diagnostics (Basel). 2022. PMID: 35453931 Free PMC article.
References
-
- Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. https://doi.org/10.1126/science.1099314. - DOI - PubMed
-
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. https://doi.org/10.1056/NEJMoa040938. - DOI - PubMed
-
- Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. https://doi.org/10.1056/NEJMoa0810699. - DOI - PubMed
-
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. https://doi.org/10.1056/NEJMoa0909530. - DOI - PubMed
-
- Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomized phase 3 trial. Lancet Oncol. 2012;13:239–46. https://doi.org/10.1016/S1470-2045(11)70393-X. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

