Dendritic Cells Enhance Polyfunctionality of Adoptively Transferred T Cells That Target Cytomegalovirus in Glioblastoma
- PMID: 29093005
- PMCID: PMC5754236
- DOI: 10.1158/0008-5472.CAN-17-0469
Dendritic Cells Enhance Polyfunctionality of Adoptively Transferred T Cells That Target Cytomegalovirus in Glioblastoma
Abstract
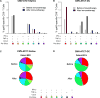
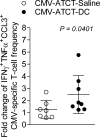
Median survival for glioblastoma (GBM) remains <15 months. Human cytomegalovirus (CMV) antigens have been identified in GBM but not normal brain, providing an unparalleled opportunity to subvert CMV antigens as tumor-specific immunotherapy targets. A recent trial in recurrent GBM patients demonstrated the potential clinical benefit of adoptive T-cell therapy (ATCT) of CMV phosphoprotein 65 (pp65)-specific T cells. However, ex vivo analyses from this study found no change in the capacity of CMV pp65-specific T cells to gain multiple effector functions or polyfunctionality, which has been associated with superior antitumor efficacy. Previous studies have shown that dendritic cells (DC) could further enhance tumor-specific CD8+ T-cell polyfunctionality in vivo when administered as a vaccine. Therefore, we hypothesized that vaccination with CMV pp65 RNA-loaded DCs would enhance the frequency of polyfunctional CMV pp65-specific CD8+ T cells after ATCT. Here, we report prospective results of a pilot trial in which 22 patients with newly diagnosed GBM were initially enrolled, of which 17 patients were randomized to receive CMV pp65-specific T cells with CMV-DC vaccination (CMV-ATCT-DC) or saline (CMV-ATCT-saline). Patients who received CMV-ATCT-DC vaccination experienced a significant increase in the overall frequencies of IFNγ+, TNFα+, and CCL3+ polyfunctional, CMV-specific CD8+ T cells. These increases in polyfunctional CMV-specific CD8+ T cells correlated (R = 0.7371, P = 0.0369) with overall survival, although we cannot conclude this was causally related. Our data implicate polyfunctional T-cell responses as a potential biomarker for effective antitumor immunotherapy and support a formal assessment of this combination approach in a larger randomized study.Significance: A randomized pilot trial in patients with GBM implicates polyfunctional T-cell responses as a biomarker for effective antitumor immunotherapy. Cancer Res; 78(1); 256-64. ©2017 AACR.
Trial registration: ClinicalTrials.gov NCT00693095.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures



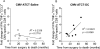
Similar articles
-
Recognition and killing of autologous, primary glioblastoma tumor cells by human cytomegalovirus pp65-specific cytotoxic T cells.Clin Cancer Res. 2014 May 15;20(10):2684-94. doi: 10.1158/1078-0432.CCR-13-3268. Epub 2014 Mar 21. Clin Cancer Res. 2014. PMID: 24658154 Free PMC article.
-
Ex vivo functional analysis, expansion and adoptive transfer of cytomegalovirus-specific T-cells in patients with glioblastoma multiforme.Immunol Cell Biol. 2012 Oct;90(9):872-80. doi: 10.1038/icb.2012.19. Epub 2012 Apr 17. Immunol Cell Biol. 2012. PMID: 22508289
-
Generation of polyclonal CMV-specific T cells for the adoptive immunotherapy of glioblastoma.J Immunother. 2012 Feb-Mar;35(2):159-68. doi: 10.1097/CJI.0b013e318247642f. J Immunother. 2012. PMID: 22306904 Free PMC article.
-
Novel mechanisms and approaches in immunotherapy for brain tumors.Discov Med. 2015 Jul-Aug;20(108):7-15. Discov Med. 2015. PMID: 26321082 Review.
-
Emerging immunotherapies for glioblastoma.Expert Opin Emerg Drugs. 2016 Jun;21(2):133-45. doi: 10.1080/14728214.2016.1186643. Expert Opin Emerg Drugs. 2016. PMID: 27223671 Free PMC article. Review.
Cited by
-
Cytomegalovirus infection of glioblastoma cells leads to NF-κB dependent upregulation of the c-MET oncogenic tyrosine kinase.Cancer Lett. 2021 Aug 10;513:26-35. doi: 10.1016/j.canlet.2021.05.005. Epub 2021 May 12. Cancer Lett. 2021. PMID: 33989707 Free PMC article.
-
Cytomegalovirus and Glioblastoma: A Review of the Biological Associations and Therapeutic Strategies.J Clin Med. 2022 Sep 4;11(17):5221. doi: 10.3390/jcm11175221. J Clin Med. 2022. PMID: 36079151 Free PMC article. Review.
-
New Approaches to Glioblastoma.Annu Rev Med. 2022 Jan 27;73:279-292. doi: 10.1146/annurev-med-042420-102102. Epub 2021 Oct 19. Annu Rev Med. 2022. PMID: 34665646 Free PMC article. Review.
-
Targeting the dendritic cell-T cell axis to develop effective immunotherapies for glioblastoma.Front Immunol. 2023 Oct 20;14:1261257. doi: 10.3389/fimmu.2023.1261257. eCollection 2023. Front Immunol. 2023. PMID: 37928547 Free PMC article. Review.
-
Prognostic value and immune cell infiltration of hypoxic phenotype-related gene signatures in glioblastoma microenvironment.J Cell Mol Med. 2020 Nov;24(22):13235-13247. doi: 10.1111/jcmm.15939. Epub 2020 Oct 3. J Cell Mol Med. 2020. PMID: 33009892 Free PMC article.
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. - PubMed
-
- Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62:3347–50. - PubMed
-
- Crough T, Beagley L, Smith C, Jones L, Walker DG, Khanna R. Ex vivo functional analysis, expansion and adoptive transfer of cytomegalovirus-specific T-cells in patients with glioblastoma multiforme. Immunol Cell Biol. 2012;90:872–80. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

