Are some people at increased risk of paracetamol-induced liver injury? A critical review of the literature
- PMID: 29067481
- PMCID: PMC5765191
- DOI: 10.1007/s00228-017-2356-6
Are some people at increased risk of paracetamol-induced liver injury? A critical review of the literature
Abstract
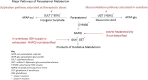
Purpose: Paracetamol is one of the world's most commonly used drugs. In overdose, it is well established to be hepatotoxic. The aim of this review was to identify factors that have been, or actually are, associated with the development of liver injury after paracetamol exposure in humans.
Method: Google Scholar and PubMed were searched on various dates between December 2016 and March 2017. Papers identified had their references analysed for further studies that might be relevant.
Results: At the time of writing, there was little good quality clinical evidence-from studies of paracetamol overdose or therapeutic use-to suggest that any groups of people are relatively protected from, or are at greater risk of, liver injury. The factors that were historically used to indicate higher risk in the UK have no good quality clinical evidence to support their re-introduction into clinical practice. The safe (and still effective) oral dose of paracetamol in patients weighing less than 50 kg has not been established.
Conclusion: There is no patient group that is unequivocally at elevated risk of paracetamol-induced liver toxicity. We propose two clinical scenarios that warrant further research. Firstly, there is a need to establish whether the dose of paracetamol should be reduced in patients with low body weight. Secondly, if or when genomic information regarding individual patients becomes readily available to inform prescribing, we propose the contribution of the genome to paracetamol toxicity should be re-investigated with robustly designed studies. Such studies could enhance the safe use of one of the most frequently taken drugs.
Keywords: Acetaminophen; DILI; Genomics; Hepatotoxicity; Paracetamol.
Conflict of interest statement
Dr. Dear is Chief Investigator on a phase 1 commercial-funded clinical trial of an Investigational Medicinal Product for paracetamol overdose (NCT03177395).
Figures
Similar articles
-
Unexpected paracetamol (acetaminophen) hepatotoxicity at standard dosage in two older patients: time to rethink 1 g four times daily?Age Ageing. 2016 Jul;45(4):566-7. doi: 10.1093/ageing/afw067. Epub 2016 Apr 21. Age Ageing. 2016. PMID: 27103600
-
Management of paracetamol overdose: current controversies.Drug Saf. 2001;24(7):503-12. doi: 10.2165/00002018-200124070-00003. Drug Saf. 2001. PMID: 11444723 Review.
-
Hepatotoxicity of therapeutic short-course paracetamol in hospital inpatients: impact of ageing and frailty.J Clin Pharm Ther. 2011 Jun;36(3):327-35. doi: 10.1111/j.1365-2710.2010.01193.x. J Clin Pharm Ther. 2011. PMID: 21545612
-
Hepatotoxicity of paracetamol and related fatalities.Eur Rev Med Pharmacol Sci. 2017 Mar;21(1 Suppl):95-101. Eur Rev Med Pharmacol Sci. 2017. PMID: 28379590
-
Stratification of paracetamol overdose patients using new toxicity biomarkers: current candidates and future challenges.Expert Rev Clin Pharmacol. 2014 Mar;7(2):181-9. doi: 10.1586/17512433.2014.880650. Epub 2014 Jan 22. Expert Rev Clin Pharmacol. 2014. PMID: 24450481 Review.
Cited by
-
Potential deleterious effects of paracetamol dose regime used in Nigeria versus that of the United States of America.Toxicol Rep. 2022 Apr 27;9:1035-1044. doi: 10.1016/j.toxrep.2022.04.025. eCollection 2022. Toxicol Rep. 2022. PMID: 36561959 Free PMC article. Review.
-
Late N-acetylcysteine for successful recovery of acetaminophen-related acute liver failure: A case report.Clin Case Rep. 2023 Sep 25;11(9):e7946. doi: 10.1002/ccr3.7946. eCollection 2023 Sep. Clin Case Rep. 2023. PMID: 37767143 Free PMC article.
-
Comparative Study of Protective Effect of Cimetidine and Verapamil on Paracetamol-Induced Hepatotoxicity in Mice.Int J Hepatol. 2020 Jan 23;2020:9185361. doi: 10.1155/2020/9185361. eCollection 2020. Int J Hepatol. 2020. PMID: 32099681 Free PMC article.
-
Unconjugated Hyperbilirubinemia in Acetaminophen-Related Acute Liver Failure.Am J Case Rep. 2024 Mar 22;25:e942703. doi: 10.12659/AJCR.942703. Am J Case Rep. 2024. PMID: 38514990 Free PMC article.
-
Acetaminophen-Induced Hepatotoxicity in Obesity and Nonalcoholic Fatty Liver Disease: A Critical Review.Livers. 2023 Mar;3(1):33-53. doi: 10.3390/livers3010003. Epub 2023 Jan 12. Livers. 2023. PMID: 36713231 Free PMC article.
References
-
- Ward B, Alexander-Williams JM. Paracetamol revisited: a review of the pharmacokinetics and pharmacodynamics. Acute Pain. 1999;2:139–149. doi: 10.1016/S1366-0071(99)80006-0. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical