Comprehensive assessment of the safety of olodaterol 5 µg in the Respimat® device for maintenance treatment of COPD: comparison with the long-acting β2-agonist formoterol
- PMID: 29061968
- PMCID: PMC5653794
- DOI: 10.1038/s41533-017-0059-1
Comprehensive assessment of the safety of olodaterol 5 µg in the Respimat® device for maintenance treatment of COPD: comparison with the long-acting β2-agonist formoterol
Abstract
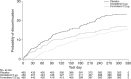
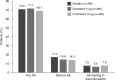
This analysis provides a comprehensive clinical assessment of the long-term safety of the licensed dose of olodaterol (5 µg once daily [QD] via Respimat® inhaler) in patients with chronic obstructive pulmonary disease by exploring the occurrence of acknowledged side effects of long-acting β2-agonists as well as those included in the olodaterol and formoterol labels. We analysed pooled data from two replicate, double-blind studies of olodaterol (5 µg QD via Respimat®) compared to formoterol (12 µg twice daily [BID]) or placebo over 48 weeks (1222.13, NCT00793624; 1222.14, NCT00796653). Patients could continue their background treatment. The analysis considered adverse events (AEs) typically associated with β2-agonists, including cardiovascular events, as well as administration-related events. Descriptive statistics were provided for the incidence of AEs and aggregated AEs. The analysis included 1379 patients: 460 placebo, 459 olodaterol and 460 formoterol; AEs were reported by 70.9, 71.7 and 69.1% of patients, respectively. Exposure-adjusted incidence rates of cardiac AEs (arrhythmia and myocardial ischaemia) and cough were numerically lower in the olodaterol group than the formoterol group, while nasopharyngitis, throat irritation, metabolism and psychiatric disorders were numerically higher in the olodaterol group. The most frequent event in the olodaterol group was nasopharyngitis (placebo 8.0%; olodaterol 12.9%; formoterol 10.0%). Except for cough (incidence rate ratio of 0.46 [95% confidence interval 0.24, 0.89] in favour of olodaterol), there were no significant differences between active groups. In conclusion, olodaterol 5 µg QD was well tolerated over 48 weeks with a typical β2-agonist safety profile comparable to formoterol 12 µg BID.
Conflict of interest statement
A.K. has received a grant from Actelion Pharmaceuticals and has taken part in congresses for Boehringer Ingelheim, Almirall, Bayer, TEVA, Roche, Actelion and Novartis. H.W. reports personal fees for consulting and compensation for his institution during the conduct of the study from Boehringer Ingelheim related to the submitted work, and participation in advisory boards for Almirall, AstraZeneca, Boehringer Ingelheim and GlaxoSmithKline, in lectures for Almirall, AstraZeneca, Boehringer Ingelheim, BerlinChemie, GlaxoSmithKline, Novartis and Chiesi, and congress travel support from GlaxoSmithKline and Novartis outside the submitted work; his institution has also received compensation for the conduct of clinical studies from Almirall, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Chiesi, Takeda, AB2BIO, Bayer and Intermune outside the submitted work. M.R.M.-Y. declares that he has no competing financial interests. U.B., K.T. and F.V. are employees of Boehringer Ingelheim. L.M. reports personal fees from Applied Clinical Intelligence during the conduct of the study, grants from Asthma UK, NI Chest Heart & Stroke, NC3Rs, British Heart Foundation and Chiesi, travel and subsistence for attendance at scientific meetings from Boehringer Ingelheim, GlaxoSmithKline and Chiesi, and advisory board/consultancy fees from Almirall, NAPP, GlaxoSmithKline and Boehringer Ingelheim outside the submitted work.
Figures

Similar articles
-
One-Year Safety of Olodaterol Once Daily via Respimat® in Patients with GOLD 2-4 Chronic Obstructive Pulmonary Disease: Results of a Pre-Specified Pooled Analysis.COPD. 2015;12(5):484-93. doi: 10.3109/15412555.2014.991864. Epub 2015 Feb 18. COPD. 2015. PMID: 25692310 Free PMC article. Clinical Trial.
-
Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat® versus placebo and formoterol twice daily in patients with GOLD 2-4 COPD: results from two replicate 48-week studies.Int J Chron Obstruct Pulmon Dis. 2014 Jul 5;9:697-714. doi: 10.2147/COPD.S62502. eCollection 2014. Int J Chron Obstruct Pulmon Dis. 2014. PMID: 25045258 Free PMC article. Clinical Trial.
-
Efficacy and safety of the long-acting β2-agonist olodaterol over 4 weeks in Japanese patients with chronic obstructive pulmonary disease.Int J Chron Obstruct Pulmon Dis. 2015 Aug 20;10:1673-83. doi: 10.2147/COPD.S86002. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 26316741 Free PMC article. Clinical Trial.
-
Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2015 Oct 22;(10):CD008989. doi: 10.1002/14651858.CD008989.pub3. Cochrane Database Syst Rev. 2015. PMID: 26490945 Review.
-
Striving for optimal bronchodilation: focus on olodaterol.Int J Chron Obstruct Pulmon Dis. 2016 Mar 1;11:439-44. doi: 10.2147/COPD.S96070. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27042036 Free PMC article. Review.
Cited by
-
Comparison of Effectiveness Using Different Dual Bronchodilator Agents in Chronic Obstructive Pulmonary Disease Treatment.J Clin Med. 2021 Jun 16;10(12):2649. doi: 10.3390/jcm10122649. J Clin Med. 2021. PMID: 34208599 Free PMC article.
-
Cardiovascular events and all-cause mortality in patients with chronic obstructive pulmonary disease using olodaterol and other long-acting beta2-agonists.Pharmacoepidemiol Drug Saf. 2022 Aug;31(8):827-839. doi: 10.1002/pds.5432. Epub 2022 May 13. Pharmacoepidemiol Drug Saf. 2022. PMID: 35320605 Free PMC article.
-
Tiotropium/olodaterol versus tiotropium in Japanese patients with COPD: results from the DYNAGITO study.Int J Chron Obstruct Pulmon Dis. 2018 Jul 13;13:2147-2156. doi: 10.2147/COPD.S169941. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 30034230 Free PMC article. Clinical Trial.
-
No Influence on Cardiac Arrhythmia or Heart Rate from Long-Term Treatment with Tiotropium/Olodaterol versus Monocomponents by Holter ECG Analysis in Patients with Moderate-to-Very-Severe COPD.Int J Chron Obstruct Pulmon Dis. 2020 Aug 10;15:1945-1953. doi: 10.2147/COPD.S246350. eCollection 2020. Int J Chron Obstruct Pulmon Dis. 2020. PMID: 32848380 Free PMC article.
-
Relationship of inhaled long-acting bronchodilators with cardiovascular outcomes among patients with stable COPD: a meta-analysis and systematic review of 43 randomized trials.Int J Chron Obstruct Pulmon Dis. 2019 Apr 11;14:799-808. doi: 10.2147/COPD.S198288. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 31114181 Free PMC article.
References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, updated 2016. http://www.goldcopd.org/ (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

