p16, pRb, and p53 in Feline Oral Squamous Cell Carcinoma
- PMID: 29056726
- PMCID: PMC5606583
- DOI: 10.3390/vetsci3030018
p16, pRb, and p53 in Feline Oral Squamous Cell Carcinoma
Abstract
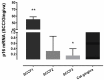
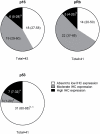
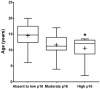
Feline oral squamous cell carcinoma (FOSCC) is a highly aggressive head and neck cancer in cats, but the molecular pathogenesis of this cancer is still uncertain. In this study, p16, p53, and pRb proteins were detected and quantified by immunohistochemistry in forty-three FOSCC primary tumors and three FOSCC xenografts. p16 mRNA levels were also measured in three FOSCC cell lines (SCCF1, F2, and F3), which were consistent with their p16 immunoreactivity. Feline SCCF1 cells had very high levels of p16 protein and mRNA (55-fold greater) compared to SCCF2 and F3. A partial feline p16 cDNA sequence was amplified and sequenced. The average age of cats with FOSCC with high p16 immunoreactivity was significantly lower than the average age in the low p16 group. Eighteen of 43 (42%) FOSCCs had low p16 intensity, while 6/43 (14%) had high p16 immunoreactivity. Feline papillomavirus L1 (major capsid) DNA was not detected in the SCC cell lines or the FOSCCs with high p16 immunostaining. Five of 6 (83%) of the high p16 FOSCC had low p53, but only 1/6 (17%) had low pRb immunoreactivity. In summary, the staining pattern of p16, p53, and pRb in FOSCC was different from human head and neck squamous cell carcinoma and feline cutaneous squamous cell carcinoma. The majority of FOSCCs have low p16 immunostaining intensity, therefore, inactivation of CDKN2A is suspected to play a role in the pathogenesis of FOSCC. A subset of FOSCCs had increased p16 protein, which supports an alternate pathogenesis of cancer in these cats.
Keywords: feline; immunohistochemistry; oral; p16; p53; pRb; squamous cell carcinoma.
Conflict of interest statement
All authors declared no potential conflicts of interest including financial, personal or other relationships with other people or organizations that could inappropriately influence, or be perceived to influence the research, authorship, and/or publication of this article. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures




Similar articles
-
Feline Oral Squamous Cell Carcinoma: A Critical Review of Etiologic Factors.Vet Sci. 2022 Oct 11;9(10):558. doi: 10.3390/vetsci9100558. Vet Sci. 2022. PMID: 36288171 Free PMC article. Review.
-
A virome sequencing approach to feline oral squamous cell carcinoma to evaluate viral causative factors.Vet Microbiol. 2020 Jan;240:108491. doi: 10.1016/j.vetmic.2019.108491. Epub 2019 Nov 2. Vet Microbiol. 2020. PMID: 31902496 Free PMC article.
-
Expression of mPGES1 and p16 in feline and human oral squamous cell carcinoma: A comparative oncology approach.Vet Comp Oncol. 2024 Jun;22(2):204-216. doi: 10.1111/vco.12967. Epub 2024 Feb 20. Vet Comp Oncol. 2024. PMID: 38378135
-
Loss of retinoblastoma protein, but not p53, is associated with the presence of papillomaviral DNA in feline viral plaques, Bowenoid in situ carcinomas, and squamous cell carcinomas.Vet Pathol. 2012 May;49(3):538-45. doi: 10.1177/0300985811419534. Epub 2011 Sep 19. Vet Pathol. 2012. PMID: 21930804
-
Animal models of head and neck squamous cell carcinoma.Vet J. 2016 Apr;210:7-16. doi: 10.1016/j.tvjl.2015.11.006. Epub 2015 Nov 24. Vet J. 2016. PMID: 26965084 Review.
Cited by
-
Feline Oral Squamous Cell Carcinoma: A Critical Review of Etiologic Factors.Vet Sci. 2022 Oct 11;9(10):558. doi: 10.3390/vetsci9100558. Vet Sci. 2022. PMID: 36288171 Free PMC article. Review.
-
Utilizing feline oral squamous cell carcinoma patients to develop NQO1-targeted therapy.Neoplasia. 2021 Aug;23(8):811-822. doi: 10.1016/j.neo.2021.06.008. Epub 2021 Jul 8. Neoplasia. 2021. PMID: 34246985 Free PMC article.
-
A virome sequencing approach to feline oral squamous cell carcinoma to evaluate viral causative factors.Vet Microbiol. 2020 Jan;240:108491. doi: 10.1016/j.vetmic.2019.108491. Epub 2019 Nov 2. Vet Microbiol. 2020. PMID: 31902496 Free PMC article.
-
Emerging tyrosine kinase inhibitors for head and neck cancer.Expert Opin Emerg Drugs. 2022 Sep;27(3):333-344. doi: 10.1080/14728214.2022.2125954. Epub 2022 Sep 21. Expert Opin Emerg Drugs. 2022. PMID: 36131561 Free PMC article. Review.
-
Prevalence of p53 dysregulations in feline oral squamous cell carcinoma and non-neoplastic oral mucosa.PLoS One. 2019 Apr 18;14(4):e0215621. doi: 10.1371/journal.pone.0215621. eCollection 2019. PLoS One. 2019. PMID: 30998743 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

