Yeast Killer Toxin K28: Biology and Unique Strategy of Host Cell Intoxication and Killing
- PMID: 29053588
- PMCID: PMC5666379
- DOI: 10.3390/toxins9100333
Yeast Killer Toxin K28: Biology and Unique Strategy of Host Cell Intoxication and Killing
Erratum in
-
Correction: Becker, B. et al. Yeast Killer Toxin K28: Biology and Unique Strategy of Host Cell Intoxication and Killing.Toxins (Basel). 2018 Mar 23;10(4):132. doi: 10.3390/toxins10040132. Toxins (Basel). 2018. PMID: 29570623 Free PMC article. No abstract available.
Abstract
The initial discovery of killer toxin-secreting brewery strains of Saccharomyces cerevisiae (S. cerevisiae) in the mid-sixties of the last century marked the beginning of intensive research in the yeast virology field. So far, four different S. cerevisiae killer toxins (K28, K1, K2, and Klus), encoded by cytoplasmic inherited double-stranded RNA viruses (dsRNA) of the Totiviridae family, have been identified. Among these, K28 represents the unique example of a yeast viral killer toxin that enters a sensitive cell by receptor-mediated endocytosis to reach its intracellular target(s). This review summarizes and discusses the most recent advances and current knowledge on yeast killer toxin K28, with special emphasis on its endocytosis and intracellular trafficking, pointing towards future directions and open questions in this still timely and fascinating field of killer yeast research.
Keywords: A/B toxin; H/KDEL receptor; K28; S. cerevisiae; cell cycle arrest; cell wall receptor; killer toxin; retrograde protein transport; retrotranslocation; toxin immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
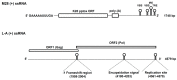
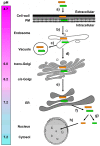
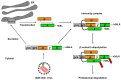
 ) and the connecting disulfide (s-s) between α and β is formed by protein disulfide isomerase (Pdi1p); (c) In the cis-Golgi, the furin-like endopeptidase, Kex2p, removes the pro-sequence and γ-subunit which leads to a disulfide-bonded α/β heterodimer. Although the precise function of the pro-region is still not fully understood, it has been proposed to be important for proper post-translational pptox import into the ER lumen [40]. While the inter-chain disulfide in the heterodimer has clearly been demonstrated to be positioned between the single cysteine in α (Cys56) and Cys333 in β, the exact position of the cysteine residues in β that form the intra-chain disulfide and the single free thiol is still hypothetical [41,42]; (d) In the trans-Golgi, the C-terminus of the α-subunit as well as the C-terminal arginine of the β-subunit are removed by carboxypeptidase Kex1p cleavage, which unmasks the ER retention/targeting motif, HDEL, thereby converting the precursor in its biologically active conformation; (e) K28 is finally secreted as a 21 kDa heterodimer, whose β-C terminus carries a potential ER retention/targeting signal required for toxin uptake in a sensitive target cell [14].
) and the connecting disulfide (s-s) between α and β is formed by protein disulfide isomerase (Pdi1p); (c) In the cis-Golgi, the furin-like endopeptidase, Kex2p, removes the pro-sequence and γ-subunit which leads to a disulfide-bonded α/β heterodimer. Although the precise function of the pro-region is still not fully understood, it has been proposed to be important for proper post-translational pptox import into the ER lumen [40]. While the inter-chain disulfide in the heterodimer has clearly been demonstrated to be positioned between the single cysteine in α (Cys56) and Cys333 in β, the exact position of the cysteine residues in β that form the intra-chain disulfide and the single free thiol is still hypothetical [41,42]; (d) In the trans-Golgi, the C-terminus of the α-subunit as well as the C-terminal arginine of the β-subunit are removed by carboxypeptidase Kex1p cleavage, which unmasks the ER retention/targeting motif, HDEL, thereby converting the precursor in its biologically active conformation; (e) K28 is finally secreted as a 21 kDa heterodimer, whose β-C terminus carries a potential ER retention/targeting signal required for toxin uptake in a sensitive target cell [14].
Similar articles
-
Production of fluorescent and cytotoxic K28 killer toxin variants through high cell density fermentation of recombinant Pichia pastoris.Microb Cell Fact. 2017 Dec 19;16(1):228. doi: 10.1186/s12934-017-0844-0. Microb Cell Fact. 2017. PMID: 29258515 Free PMC article.
-
Relationships and Evolution of Double-Stranded RNA Totiviruses of Yeasts Inferred from Analysis of L-A-2 and L-BC Variants in Wine Yeast Strain Populations.Appl Environ Microbiol. 2017 Feb 1;83(4):e02991-16. doi: 10.1128/AEM.02991-16. Print 2017 Feb 15. Appl Environ Microbiol. 2017. PMID: 27940540 Free PMC article.
-
A yeast killer toxin screen provides insights into a/b toxin entry, trafficking, and killing mechanisms.Dev Cell. 2009 Oct;17(4):552-60. doi: 10.1016/j.devcel.2009.08.006. Dev Cell. 2009. PMID: 19853568 Free PMC article.
-
Viral induced yeast apoptosis.Biochim Biophys Acta. 2008 Jul;1783(7):1413-7. doi: 10.1016/j.bbamcr.2008.01.017. Epub 2008 Feb 7. Biochim Biophys Acta. 2008. PMID: 18291112 Review.
-
Yeast dsRNA viruses: replication and killer phenotypes.Mol Microbiol. 1991 Oct;5(10):2331-8. doi: 10.1111/j.1365-2958.1991.tb02078.x. Mol Microbiol. 1991. PMID: 1665194 Review.
Cited by
-
Saccharomyces paradoxus K66 Killer System Evidences Expanded Assortment of Helper and Satellite Viruses.Viruses. 2018 Oct 16;10(10):564. doi: 10.3390/v10100564. Viruses. 2018. PMID: 30332789 Free PMC article.
-
Dynamic modelling of the killing mechanism of action by virus-infected yeasts.J R Soc Interface. 2019 Mar 29;16(152):20190064. doi: 10.1098/rsif.2019.0064. J R Soc Interface. 2019. PMID: 30890050 Free PMC article.
-
Adaptive Response of Saccharomyces Hosts to Totiviridae L-A dsRNA Viruses Is Achieved through Intrinsically Balanced Action of Targeted Transcription Factors.J Fungi (Basel). 2022 Apr 9;8(4):381. doi: 10.3390/jof8040381. J Fungi (Basel). 2022. PMID: 35448612 Free PMC article.
-
Discovery of a rapidly evolving yeast defense factor, KTD1, against the secreted killer toxin K28.Proc Natl Acad Sci U S A. 2023 Feb 21;120(8):e2217194120. doi: 10.1073/pnas.2217194120. Epub 2023 Feb 17. Proc Natl Acad Sci U S A. 2023. PMID: 36800387 Free PMC article.
-
Molecular Basis of Yeasts Antimicrobial Activity-Developing Innovative Strategies for Biomedicine and Biocontrol.Curr Issues Mol Biol. 2024 May 14;46(5):4721-4750. doi: 10.3390/cimb46050285. Curr Issues Mol Biol. 2024. PMID: 38785553 Free PMC article. Review.
References
-
- Bevan E.A., Makower M. The physiological basis of the killer character in yeast; Proceedings of the 11th International Congress Genet; The Hague, The Netherlands. September 1963; pp. 202–203.
-
- Theisen S., Molkenau E., Schmitt M.J. Wicaltin, a new protein toxin secreted by the yeast williopsis californica and its broad-spectrum antimycotic potential. J. Microbiol. Biotechnol. 2000;10:547–550.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

